Abstract
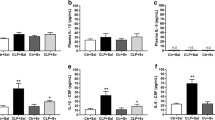
Sepsis is an organ dysfunction caused by a host’s unregulated response to infection, causing long-term brain dysfunction with microglial activation, the release of inflammatory components, and mitochondrial changes. Neuroinflammation can increase the expression of the 18-kD translocator protein (TSPO) in the mitochondria, leading to the activation of the microglia and the release of inflammatory components. The antagonist PK-11195 can modulate TSPO and reduce microglial activation and cognitive damage presented in an animal model of sepsis. The aim of this was to evaluate the effects of PK-11195 on long-term brain inflammation and cognitive impairment in an animal model of sepsis. Wistar rats, 60 days old, were submitted to cecal ligation and puncture (CLP) surgery, divided into groups control/saline, control/PK-11195, sepsis/saline, and sepsis/PK-11195. Immediately after surgery, the antagonist PK-11195 was administered at a dose of 3 mg/kg. Ten days after CLP surgery, the animals were submitted to behavioral tests and determination of brain inflammatory parameters. The sepsis/saline group presented cognitive damage. However, there was damage prevention in animals that received PK-11195. Besides, the sepsis increased the levels of cytokines and M1 microglia markers and caused oxidative damage. However, PK-11195 had the potential to decrease inflammation. These events show that the modulation of neuroinflammation during sepsis by PK-11195, possibly related to changes in TSPO, improves mitochondrial function in the animals’ brains. In conclusion, the antagonist PK-11195 attenuated brain inflammation and prevented cognitive impairment in animals subjected to sepsis.





Similar content being viewed by others
Data Availability
All the data generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR et al (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Yealy DM, Huang DT, Delaney A, Knight M, Randolph AG, Daniels R, Nutbeam T (2015) Recognizing and managing sepsis: what needs to be done? BMC Med 13:98. https://doi.org/10.1186/s12916-015-0335-2
Annane D, Sharshar T (2015) Cognitive decline after sepsis. Lancet Respir Med 3(1):61–69
Barichello T, Sayana P, Giridharan VV, Arumanayagam AS, Narendran B, Della Giustina A, Petronilho F, Quevedo J et al (2019) Long-term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol 56(1):186–251. https://doi.org/10.1007/s12035-018-1048-2
Andonegui G, Zelinski EL, Schubert CL, Knight D, Craig LA, Winston BW, Spanswick SC, Petri B et al (2018) Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight 3(9). https://doi.org/10.1172/jci.insight.99364
Nwafor DC, Brichacek AL, Mohammad AS, Griffith J, Lucke-Wold BP, Benkovic SA, Geldenhuys WJ, Lockman PR et al (2019) Targeting the blood-brain barrier to prevent sepsis-associated cognitive impairment. J Central Nervous Syst Dis 11:1179573519840652. https://doi.org/10.1177/1179573519840652
Gordon SM, Jackson JC, Ely EW, Burger C, Hopkins RO (2004) Clinical identification of cognitive impairment in ICU survivors: insights for intensivists. Intensive Care Med 30(11):1997–2008. https://doi.org/10.1007/s00134-004-2418-y
Bozza FA, D'Avila JC, Ritter C, Sonneville R, Sharshar T, Dal-Pizzol F (2013) Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 39(Suppl 1):10–16. https://doi.org/10.1097/SHK.0b013e31828fade1
Steckert AV, Comim CM, Igna DM, Dominguini D, Mendonca BP, Ornell F, Colpo GD, Gubert C et al (2015) Effects of sodium butyrate on aversive memory in rats submitted to sepsis. Neurosci Lett 595:134–138. https://doi.org/10.1016/j.neulet.2015.04.019
Steckert AV, Comim CM, Mina F, Mendonca BP, Dominguini D, Ferreira GK, Carvalho-Silva M, Vieira JS et al (2013) Late brain alterations in sepsis-survivor rats. Synapse 67(11):786–793. https://doi.org/10.1002/syn.21686
Michelon C, Michels M, Abatti M, Vieira A, Borges H, Dominguini D, Barichello T, Dal-Pizzol F (2020) The role of secretase pathway in long-term brain inflammation and cognitive impairment in an animal model of severe sepsis. Mol Neurobiol 57(2):1159–1169. https://doi.org/10.1007/s12035-019-01808-1
Manfredini A, Constantino L, Pinto MC, Michels M, Burger H, Kist LW, Silva MC, Gomes LM et al (2019) Mitochondrial dysfunction is associated with long-term cognitive impairment in an animal sepsis model. Clin Sci 133(18):1993–2004. https://doi.org/10.1042/CS20190351
Biff D, Petronilho F, Constantino L, Vuolo F, Zamora-Berridi GJ, Dall'Igna DM, Comim CM, Quevedo J et al (2013) Correlation of acute phase inflammatory and oxidative markers with long-term cognitive impairment in sepsis survivors rats. Shock 40(1):45–48. https://doi.org/10.1097/SHK.0b013e3182959cfa
Comim CM, Cassol OJ Jr, Constantino LS, Felisberto F, Petronilho F, Rezin GT, Scaini G, Daufenbach JF et al (2011) Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochem Res 36(2):304–311. https://doi.org/10.1007/s11064-010-0320-2
Tomasi CD, Vuolo F, Generoso J, Soares M, Barichello T, Quevedo J, Ritter C, Dal-Pizzol F (2017) Biomarkers of delirium in a low-risk community-acquired pneumonia-induced sepsis. Mol Neurobiol 54(1):722–726. https://doi.org/10.1007/s12035-016-9708-6
Michels M, Michelon C, Damasio D, Vitali AM, Ritter C, Dal-Pizzol F (2019) Biomarker predictors of delirium in acutely ill patients: a systematic review. J Geriatr Psychiatry Neurol 32(3):119–136. https://doi.org/10.1177/0891988719834346
Gasparotto J, Girardi CS, Somensi N, Ribeiro CT, Moreira JCF, Michels M, Sonai B, Rocha M et al (2018) Receptor for advanced glycation end products mediates sepsis-triggered amyloid-beta accumulation, Tau phosphorylation, and cognitive impairment. J Biol Chem 293(1):226–244. https://doi.org/10.1074/jbc.M117.786756
Dal-Pizzol F, Pasquali M, Quevedo J, Gelain DP, Moreira JC (2012) Is there a role for high mobility group box 1 and the receptor for advanced glycation end products in the genesis of long-term cognitive impairment in sepsis survivors? Mol Med 18:1357–1358; author reply 1359. https://doi.org/10.2119/molmed.2012.00317
Giridharan VV, Generoso JS, Collodel A, Dominguini D, Faller CJ, Tardin F, Bhatti GS, Petronilho F et al (2020) Receptor for advanced glycation end products (RAGE) mediates cognitive impairment triggered by pneumococcal meningitis. Neurotherapeutics 4(10):020–00917
Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D et al (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27(8):402–409. https://doi.org/10.1016/j.tips.2006.06.005
Gut P (2015) Targeting mitochondrial energy metabolism with TSPO ligands. Biochem Soc Trans 43(4):537–542
Zhou T, Dang Y, Zheng YH (2014) The mitochondrial translocator protein, TSPO, inhibits HIV-1 envelope glycoprotein biosynthesis via the endoplasmic reticulum-associated protein degradation pathway. J Virol 88(6):3474–3484
Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF et al (2008) Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis 32(3):412–419. https://doi.org/10.1016/j.nbd.2008.08.001
Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W et al (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 21(2):404–412. https://doi.org/10.1016/j.nbd.2005.08.002
Giridharan VV, Collodel A, Generoso JS, Scaini G, Wassather R, Selvaraj S, Hasbun R, Dal-Pizzol F et al (2020) Neuroinflammation trajectories precede cognitive impairment after experimental meningitis-evidence from an in vivo PET study. J Neuroinflammation 17(1):019–1692
Wala EP, Sloan JW, Jing X (2000) Pharmacokinetics of the peripheral benzodiazepine receptor antagonist, PK 11195, in rats. The effect of dose and gender. Pharmacol Res 41(4):461–468. https://doi.org/10.1006/phrs.1999.0617
Veiga S, Carrero P, Pernia O, Azcoitia I, Garcia-Segura LM (2007) Translocator protein 18 kDa is involved in the regulation of reactive gliosis. Glia 55(14):1426–1436. https://doi.org/10.1002/glia.20558
Monga S, Weizman A, Gavish M (2020) The efficacy of the novel TSPO ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 compared to the classical TSPO ligand PK 11195 to counteract the release of chemokines from LPS-stimulated BV-2 microglial cells. Biology 9(9):291. https://doi.org/10.3390/biology9090291
Zhou D, Ji L, Chen Y (2020) Correction to: TSPO modulates IL-4-induced microglia/macrophage M2 polarization Via PPAR-gamma pathway. J Mol Neurosci 70(7):1164. https://doi.org/10.1007/s12031-020-01571-2
Le Fur G, Vaucher N, Perrier ML, Flamier A, Benavides J, Renault C, Dubroeucq MC, Gueremy C et al (1983) Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies. Life Sci 33(5):449–457. https://doi.org/10.1016/0024-3205(83)90794-4
Klegeris A, McGeer EG, McGeer PL (2000) Inhibitory action of 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxam ide (PK 11195) on some mononuclear phagocyte functions. Biochem Pharmacol 59(10):1305–1314. https://doi.org/10.1016/s0006-2952(00)00252-5
Ma L, Zhang H, Liu N, Wang PQ, Guo WZ, Fu Q, Jiao LB, Ma YQ et al (2016) TSPO ligand PK11195 alleviates neuroinflammation and beta-amyloid generation induced by systemic LPS administration. Brain Res Bull 121:192–200. https://doi.org/10.1016/j.brainresbull.2016.02.001
Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL et al (2010) Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage 49(4):2924–2932. https://doi.org/10.1016/j.neuroimage.2009.11.056
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD (2019) Global brain inflammation in stroke. Lancet Neurol 18(11):1058–1066. https://doi.org/10.1016/S1474-4422(19)30078-X
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D et al (2010) Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9(12):971–988. https://doi.org/10.1038/nrd3295
Ye C, Lin L, Zhang P, Chen Y, Huang J, Lin X (2020) The protective effect of PK11195 on D-galactose-induced amnestic mild cognitive impairment in rats. Ann Transl Med 8(18):1190. https://doi.org/10.21037/atm-20-6157
Fink MP, Heard SO (1990) Laboratory models of sepsis and septic shock. J Surg Res 49(2):186–196. https://doi.org/10.1016/0022-4804(90)90260-9
Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH et al (2000) Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem 7(5):333–340. https://doi.org/10.1101/lm.34600
de Lima MN, Laranja DC, Bromberg E, Roesler R, Schroder N (2005) Pre- or post-training administration of the NMDA receptor blocker MK-801 impairs object recognition memory in rats. Behav Brain Res 156(1):139–143. https://doi.org/10.1016/j.bbr.2004.05.016
Roesler R, Vianna MR, de-Paris F, Quevedo J (1999) Memory-enhancing treatments do not reverse the impairment of inhibitory avoidance retention induced by NMDA receptor blockade. Neurobiol Learn Mem 72(3):252–258. https://doi.org/10.1006/nlme.1999.3910
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-i
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Pannell M, Economopoulos V, Wilson TC, Kersemans V, Isenegger PG, Larkin JR, Smart S, Gilchrist S et al (2020) Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia 68(2):280–297. https://doi.org/10.1002/glia.23716
Vignal N, Boulay AC, San C, Cohen-Salmon M, Rizzo-Padoin N, Sarda-Mantel L, Decleves X, Cisternino S et al (2020) Astroglial Connexin 43 deficiency protects against LPS-induced neuroinflammation: a TSPO brain microPET Study with [(18)F]FEPPA. Cells 9(2):389. https://doi.org/10.3390/cells9020389
Barichello T, Simoes LR, Collodel A, Giridharan VV, Dal-Pizzol F, Macedo D, Quevedo J (2017) The translocator protein (18kDa) and its role in neuropsychiatric disorders. Neurosci Biobehav Rev 83:183–199. https://doi.org/10.1016/j.neubiorev.2017.10.010
Santoro A, Mattace Raso G, Taliani S, Da Pozzo E, Simorini F, Costa B, Martini C, Laneri S et al (2016) TSPO-ligands prevent oxidative damage and inflammatory response in C6 glioma cells by neurosteroid synthesis. Eur J Pharm Sci 88:124–131. https://doi.org/10.1016/j.ejps.2016.04.006
Bae KR, Shim HJ, Balu D, Kim SR, Yu SW (2014) Translocator protein 18 kDa negatively regulates inflammation in microglia. J NeuroImmune Pharmacol 9(3):424–437. https://doi.org/10.1007/s11481-014-9540-6
Goldim MP, Danielski LG, Rodrigues JF, Joaquim L, Garbossa L, de Oliveira Junior AN, Metzker KLL, Giustina AD et al (2019) Oxidative stress in the choroid plexus contributes to blood-cerebrospinal fluid barrier disruption during sepsis development. Microvasc Res 123:19–24. https://doi.org/10.1016/j.mvr.2018.12.001
Zhou D, Ji L, Chen Y (2019) TSPO modulates IL-4-Induced microglia/macrophage M2 polarization via PPAR-gamma pathway. J Mol Neurosci 70:542–549. https://doi.org/10.1007/s12031-019-01454-1
Azrad M, Zeineh N, Weizman A, Veenman L, Gavish M (2019) The TSPO ligands 2-Cl-MGV-1, MGV-1, and PK11195 differentially suppress the inflammatory response of BV-2 microglial cell to LPS. Int J Mol Sci 20 (3). doi:https://doi.org/10.3390/ijms20030594
Zhou D, Ji L, Chen Y (2020) TSPO modulates IL-4-induced microglia/macrophage M2 polarization via PPAR-gamma pathway. J Mol Neurosci 70(4):542–549. https://doi.org/10.1007/s12031-019-01454-1
Choi HB, Khoo C, Ryu JK, van Breemen E, Kim SU, McLarnon JG (2002) Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem 83(3):546–555. https://doi.org/10.1046/j.1471-4159.2002.01122.x
Christensen A, Pike CJ (2018) TSPO ligand PK11195 improves Alzheimer-related outcomes in aged female 3xTg-AD mice. Neurosci Lett 683:7–12. https://doi.org/10.1016/j.neulet.2018.06.029
Leaver KR, Reynolds A, Bodard S, Guilloteau D, Chalon S, Kassiou M (2012) Effects of translocator protein (18 kDa) ligands on microglial activation and neuronal death in the quinolinic-acid-injected rat striatum. ACS Chem Neurosci 3(2):114–119. https://doi.org/10.1021/cn200099e
Michels M, Abatti M, Vieira A, Avila P, Goulart AI, Borges H, Corneo E, Dominguini D et al (2020) Modulation of microglial phenotypes improves sepsis-induced hippocampus-dependent cognitive impairments and decreases brain inflammation in an animal model of sepsis. Clin Sci 134(7):765–776. https://doi.org/10.1042/CS20191322
Giridharan VV, Collodel A, Generoso JS, Scaini G, Wassather R, Selvaraj S, Hasbun R, Dal-Pizzol F et al (2020) Neuroinflammation trajectories precede cognitive impairment after experimental meningitis-evidence from an in vivo PET study. J Neuroinflammation 17(1):5. https://doi.org/10.1186/s12974-019-1692-0
Michels M, Abatti MR, Avila P, Vieira A, Borges H, Carvalho Junior C, Wendhausen D, Gasparotto J et al (2020) Characterization and modulation of microglial phenotypes in an animal model of severe sepsis. J Cell Mol Med 24(1):88–97. https://doi.org/10.1111/jcmm.14606
Michels M, Avila P, Pescador B, Vieira A, Abatti M, Cucker L, Borges H, Goulart AI et al (2019) Microglial cells depletion increases inflammation and modifies microglial phenotypes in an animal model of severe sepsis. Mol Neurobiol 56(11):7296–7304. https://doi.org/10.1007/s12035-019-1606-2
Funding
This research was supported by Universidade do Extremo Sul Catarinense (UNESC) and National Institute of Science and Technology—Translational Medicine (INCT).
Author information
Authors and Affiliations
Contributions
DD, FDF, and TB designed the study. DD and JSG prepared the manuscript. FDF and TB edited the manuscript and supervised the study. DD, AVS, MRA, and JSG were responsible behavioral tests and biochemical analysis. All the authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Statement of Ethics
All the experimental procedures were performed with the approval of the Ethics Committee from Universidade do Extremo Sul Catarinense (protocol number: 001/2015-1) and conformed to international regulations.
Conflict of Interest
The authors declare that there is no conflict of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
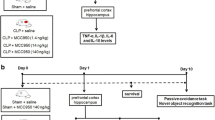
Supplemental Fig 1
- Experimental design. 60 days after birth, the rats were subjected to sepsis and treated with PK 11195. After 10 days, open field behavioral tests, recognition of novel objects, and inhibitory avoidance were performed. Soon after, the prefrontal cortex and hippocampus brain tissues were extracted for the analysis of cytokines, oxidative damage, and markers of microglial activity. (PNG 4948 kb)
Rights and permissions
About this article
Cite this article
Dominguini, D., Steckert, A.V., Abatti, M.R. et al. The Protective Effect of PK-11195 on Cognitive Impairment in Rats Survived of Polymicrobial Sepsis. Mol Neurobiol 58, 2724–2733 (2021). https://doi.org/10.1007/s12035-021-02294-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02294-0