Abstract
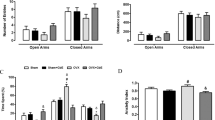
Since Ginkgo biloba extract (GbE) was reported to improve the hypothalamic serotonergic system of ovariectomized (OVX) rats, the present study aimed to verify the GbE effects on hippocampal oxidative stress, inflammation, and levels of the serotonin transporter (5-HTT), and both the serotonin (5-HT1A, 5-HT1B) and leptin receptors of OVX rats. Two-month-old female Wistar rats had their ovaries surgically removed (OVX) or not (SHAM). After 60 days, OVX rats were gavaged daily with GbE 500 mg kg−1 (OVX+GbE), while SHAM and OVX groups received saline 0.9% (vehicle) for 14 days. Rats were then euthanized, and hippocampi were collected. Both 5-HT1A and 5-HT1B levels were significantly reduced in OVX rats compared to SHAM rats, while 5-HT1A was higher in OVX+GbE rats in comparison to OVX rats. Similarly, LepR levels were increased in OVX+GbE rats compared to OVX rats, reaching similar levels to SHAM rats. Superoxide dismutase activity increased in OVX rats in relation to SHAM rats, which was restored to SHAM levels by GbE treatment. Additionally, GbE significantly increased the glutathione peroxidase activity in comparison to the SHAM group. No differences were observed either in catalase activity or in the levels of 5-HTT, PKCα, TLR-4, NF-κBp50, ERK, and CREB. In summary, our results show a potential effect of GbE on hippocampal pathways involved in feeding behavior, and thus, they suggest that GbE activity might improve menopausal-related hippocampal disorders, offering an alternative therapeutic tool particularly for women to whom hormone replacement therapy may be contraindicated.
Graphical abstract




Similar content being viewed by others
Data Availability
All data generated or analyzed in the study will be available in the repository, as well as by the corresponding author upon request.
References
Herrera AY, Mather M (2015) Actions and interactions of estradiol and glucocorticoids in cognition and the brain: Implications for aging women. Neurosci Biobehav Rev 55:36–52. https://doi.org/10.1016/j.neubiorev.2015.04.005
Xu Y, López M (2018) Central regulation of energy metabolism by estrogens. Mol Metab 15:104–115. https://doi.org/10.1016/j.molmet.2018.05.012
McEwen BS (2001) Invited review: estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol 91:2785–2801. https://doi.org/10.1152/jappl.2001.91.6.2785
Luine VN (2014) Estradiol and cognitive function: past, present and future. Horm Behav 66:602–618. https://doi.org/10.1016/j.yhbeh.2014.08.011
Al Abed AS, Sellami A, Brayda-Bruno L et al (2016) Estradiol enhances retention but not organization of hippocampus-dependent memory in intact male mice. Psychoneuroendocrinology 69:77–89. https://doi.org/10.1016/j.psyneuen.2016.03.014
Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J (2000) The estrogen replacement therapy of the Women’s Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer’s disease. Maturitas 34:S35–S52. https://doi.org/10.1016/S0378-5122(00)00107-9
Au A, Feher A, McPhee L, Jessa A, Oh S, Einstein G (2016) Estrogens, inflammation and cognition. Front Neuroendocrinol 40:87–100. https://doi.org/10.1016/j.yfrne.2016.01.002
Inagaki T, Gautreaux C, Luine V (2010) Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav 58:415–426. https://doi.org/10.1161/CIRCULATIONAHA.110.956839
Christensen A, Pike CJ (2015) Menopause, obesity and inflammation: interactive risk factors for Alzheimer’s disease. Front Aging Neurosci 7:1–14. https://doi.org/10.3389/fnagi.2015.00130
Doshi SB (2013) Agarwal A. The role of oxidative stress in menopause 4:140–146. https://doi.org/10.4103/0976-7800.118990
Sorpreso ICE, Soares Júnior JM, da Fonseca AM, Baracat EC (2015) Female aging. Rev Assoc Med Bras 61:553–556. https://doi.org/10.1590/1806-9282.61.06.553
Li LH, Wang ZC, Yu J, Zhang YQ (2014) Ovariectomy results in variable changes in nociception, mood and depression in adult female rats. PLoS One 9:1–11. https://doi.org/10.1371/journal.pone.0094312
Del Río JP, Alliende MI, Molina N et al (2018) Steroid hormones and their action in women’s brains: the importance of hormonal balance. Front Public Heal 6:1–15. https://doi.org/10.3389/fpubh.2018.00141
Dornellas APS, Boldarine VT, Pedroso AP, Carvalho LOT, de Andrade IS, Vulcani-Freitas TM, dos Santos CCC, do Nascimento CMPO et al (2018) High-fat feeding improves anxiety-type behavior induced by ovariectomy in rats. Front Neurosci 12:1–13. https://doi.org/10.3389/fnins.2018.00557
Banin RM, Machado MMF, Telles MM (2017) A bi-directional relation between menopause and obesity: focus on the main causes and associated metabolic diseases. Curr res diabetes Obes J 3:59–61. https://doi.org/10.19080/CRDOJ.2017.3.555609 curre
Kanoski SE, Grill HJ (2017) Review hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry 81:748–756. https://doi.org/10.1016/j.biopsych.2015.09.011
Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, Grill HJ (2011) Hippocampal Leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 36:1859–1870. https://doi.org/10.1038/npp.2011.70
Epperson CN, Amin Z, Rupaurel K et al (2012) NIH public access. Psychoneuroendocrinology 37:372–382. https://doi.org/10.1016/j.psyneuen.2011.07.007.Interactive
Compton J, Van Amelsvoort T, Murphy D (2001) HRT and its effect on normal ageing of the brain and dementia. Br J Clin Pharmacol 52:647–653. https://doi.org/10.1046/j.0306-5251.2001.01492.x
Seumeren IV (2000) Weight gain and hormone replacement therapy: are women’s fears justified? Maturitas 34:3–8. https://doi.org/10.1016/S0378-5122(99)00073-0
Hirata BKS, Banin RM, Dornellas APS, de Andrade IS, Zemdegs JCS, Caperuto LC, Oyama LM, Ribeiro EB et al (2015) Ginkgo biloba extract improves insulin signaling and attenuates inflammation in retroperitoneal adipose tissue depot of obese rats. Mediat Inflamm 2015:1–9. https://doi.org/10.1155/2015/419106
Hirata BKS, Pedroso AP, Machado MMF, Neto NIP, Perestrelo BO, de Sá RDCC, Alonso-Vale MIC, Nogueira FN et al (2019) Ginkgo biloba extract modulates the retroperitoneal fat depot proteome and reduces oxidative stress in diet-induced obese rats. Front Pharmacol 10:1–11. https://doi.org/10.3389/fphar.2019.00686
Kennedy D, Wightman E (2011) British pharmacopeia. Adv Nutr 2:32–50. https://doi.org/10.3945/an.110.000117.32
Heinonen T, Gaus W (2015) Cross matching observations on toxicological and clinical data for the assessment of tolerability and safety of Ginkgo biloba leaf extract. Toxicology 327:95–115. https://doi.org/10.1016/j.tox.2014.10.013
Banin RM, Hirata BKS, Andrade IS, Zemdegs JCS, Clemente APG, Dornellas APS, Boldarine VT, Estadella D et al (2014) Beneficial effects of Ginkgo biloba extract on insulin signaling cascade, dyslipidemia, and body adiposity of diet-induced obese rats. Brazilian J Med Biol Res 47:780–788. https://doi.org/10.1590/1414-431X20142983
Hirata BKS, Cruz MM, De Sá RDCC et al (2019) Potential anti-obesogenic effects of Ginkgo biloba observed in epididymal white adipose tissue of obese rats. Front Endocrinol (Lausanne) 10:1–11. https://doi.org/10.3389/fendo.2019.00284
Ding S, Dudley E, Plummer S, Tang J, Newton RP, Brenton AG (2006) Quantitative determination of major active components in Ginkgo biloba dietary supplements by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 20:2753–2760. https://doi.org/10.1002/rcm.2646
Banin RM, de Andrade IS, Cerutti SM, Oyama LM, Telles MM, Ribeiro EB (2017) Ginkgo biloba extract (GbE) stimulates the hypothalamic serotonergic system and attenuates obesity in ovariectomized rats. Front Pharmacol 8:1–11. https://doi.org/10.3389/fphar.2017.00605
Spijker S (2011) Neuroproteomics: dissection of rodent brain regions. Neuromethods 57:13–27. https://doi.org/10.1007/978-1-61779-111-6
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Lovejoy JC, Champagne CM, De Jonge L et al (2008) Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 32:949–958. https://doi.org/10.1038/ijo.2008.25
Al-Safi ZA, Polotsky AJ (2015) Obesity and menopause. Best Pract Res Clin Obstet Gynaecol 29:548–553. https://doi.org/10.1016/j.bpobgyn.2014.12.002
Boldarine VT, Pedroso AP, Neto NIP, Dornellas APS, Nascimento CMO, Oyama LM, Ribeiro EB (2019) High-fat diet intake induces depressive-like behavior in ovariectomized rats. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-47152-1
Hirata BKS, Cruz MM, De Sá RDCC et al (2019) Potential anti-obesogenic effects of Ginkgo biloba observed in epididymal white adipose tissue of obese rats. Front Endocrinol (Lausanne) 10:10. https://doi.org/10.3389/fendo.2019.00284
Oliveira DR, Sanada PF, Filho ACS, Conceição GMS, Cerutti JM, Cerutti SM (2013) Long-term treatment with standardized extract of Ginkgo biloba L. enhances the conditioned suppression of licking in rats by the modulation of neuronal and glial cell function in the dorsal hippocampus and central amygdala. Neuroscience 235:70–86. https://doi.org/10.1016/j.neuroscience.2013.01.009
Ribeiro ML, Moreira LM, Arçari DP, dos Santos LF, Marques AC, Pedrazzoli J Jr, Cerutti SM (2016) Protective effects of chronic treatment with a standardized extract of Ginkgo biloba L. in the prefrontal cortex and dorsal hippocampus of middle-aged rats. Behav Brain Res 313:144–150. https://doi.org/10.1016/j.bbr.2016.06.029
Gaiardo RB, Abreu TF, Tashima AK, Telles MM, Cerutti SM (2019) Target proteins in the dorsal hippocampal formation sustain the memory-enhancing and neuroprotective effects of Ginkgo biloba. Front Pharmacol 9:1–14. https://doi.org/10.3389/fphar.2018.01533
Zamberlam CR, Tilger MAS, Moraes L, Cerutti JM, Cerutti SM (2019) Ginkgo biloba treatments reverse the impairment of conditioned suppression acquisition induced by GluN2B-NMDA and 5-HT1A receptor blockade: modulatory effects of the circuitry of the dorsal hippocampal formation. Physiol Behav 209:1–20. https://doi.org/10.1016/j.physbeh.2019.04.023
Voigt JP, Fink H (2015) Serotonin controlling feeding and satiety. Behav Brain Res 277:14–31. https://doi.org/10.1016/j.bbr.2014.08.065
Butt I, Hong A, Di J et al (2014) The effects of serotonin1A receptor on female mice body weight and food intake are associated with the differential expression of hypothalamic neuropeptides and the GABAA receptor. Neuropeptides 48:313–318. https://doi.org/10.1016/j.npep.2014.07.003
Van Doorn C, Macht VA, Grillo CA, Reagan LP (2017) Leptin resistance and hippocampal behavioral deficits. Physiol Behav 176:207–213. https://doi.org/10.1016/j.physbeh.2017.03.002
Donovan MH, Tecott LH (2013) Serotonin and the regulation of mammalian energy balance. Front Neurosci 7:1–15. https://doi.org/10.3389/fnins.2013.00036
Telles MM, Guimarães RB, Ribeiro EB (2003) Effect of leptin on the acute feeding-induced hypothalamic serotonergic stimulation in normal rats. Regul Pept 115:11–18. https://doi.org/10.1016/S0167-0115(03)00129-0
Rozin P, Dow S, Moscovitch M, Rajaram S (1998) What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychol Sci 9:392–396. https://doi.org/10.1111/1467-9280.00073
Stevenson RJ, Francis HM (2017) The hippocampus and the regulation of human food intake. Psychol Bull 143:1011–1032. https://doi.org/10.1037/bul0000109
Banin RM, Machado MMF, de Andrade IS, et al (2021) Ginkgo biloba extract (GbE) attenuates obesity and anxiety/depressive-like behaviours induced by ovariectomy. Sci Rep 11: 1-14. https://doi.org/10.1038/s41598-020-78528-3
Oliveira DR, Sanada PF, Saragossa FAC, Innocenti LR, Oler G, Cerutti JM, Cerutti SM (2009) Neuromodulatory property of standardized extract Ginkgo biloba L. (EGb 761) on memory: behavioral and molecular evidence. Brain Res 1269:68–89. https://doi.org/10.1016/j.brainres.2008.11.105
Frick KM, Tuscher JJ, Koss WA, Kim J, Taxier LR (2018) Estrogenic regulation of memory consolidation: a look beyond the hippocampus, ovaries, and females. Physiol Behav 187:57–66. https://doi.org/10.1016/j.physbeh.2017.07.028
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:1–15. https://doi.org/10.1017/jns.2016.41
Feng Z, Sun Q, Chen W, Bai Y, Hu D, Xie X (2019) The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: a literature review. Mol Med 25:1–8. https://doi.org/10.1186/s10020-019-0125-y
Mu L, Kou J, Zhu D, Yu B (2007) Comparison of neuroprotective effects of flavonoids, terpenoids, and their combinations from Ginkgo biloba on ischemia-reperfusion - injured mice. Pharm Biol 45:728–733. https://doi.org/10.1080/13880200701575486
Sagredo A, del Campo L, Martorell A, Navarro R, Martín MC, Blanco-Rivero J, Ferrer M (2013) Ovariectomy increases the participation of hyperpolarizing mechanisms in the relaxation of rat aorta. PLoS One 8:1–11. https://doi.org/10.1371/journal.pone.0073474
Behr GA, Schnorr CE, Simoes-Pires A et al (2012) Increased cerebral oxidative damage and decreased antioxidant defenses in ovariectomized and sham-operated rats supplemented with vitamin a. Cell Biol Toxicol 28:317–330. https://doi.org/10.1007/s10565-012-9226-x
Monthakantirat O, Sukano W, Umehara K, Noguchi H, Chulikhit Y, Matsumoto K (2014) Effect of miroestrol on ovariectomy-induced cognitive impairment and lipid peroxidation in mouse brain. Phytomedicine 21:1249–1255. https://doi.org/10.1016/j.phymed.2014.06.012
Kohen R, Beit-Yannai E, Berry EM, Tirosh O (1999) Overall low molecular weight antioxidant activity of biological fluids and tissues by cyclic voltammetry. Methods Enzymol 300:285–296. https://doi.org/10.1016/S0076-6879(99)00135-4
Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M (2000) Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20:2175–2183. https://doi.org/10.1161/01.ATV.20.10.2175
Saso L, Firuzi O (2014) Pharmacological applications of antioxidants: lights and shadows. Curr Drug Targets 15:1177–1199. https://doi.org/10.2174/1389450115666141024113925
Huang YH, Zhang QH (2010) Genistein reduced the neural apoptosis in the brain of ovariectomised rats by modulating mitochondrial oxidative stress. Br J Nutr 104:1297–1303. https://doi.org/10.1017/S0007114510002291
Eckert A (2012) Mitochondrial effects of Ginkgo biloba extract. Int Psychogeriatrics 24:18–20. https://doi.org/10.1017/S1041610212000531
Sadowska-Krępa E, Kłapcińska B, Pokora I, Domaszewski P, Kempa K, Podgórski T (2017) Effects of six-week Ginkgo biloba supplementation on aerobic performance, blood pro/antioxidant balance, and serum brain-derived neurotrophic factor in physically active men. Nutrients 9:1–11. https://doi.org/10.3390/nu9080803
Rimbach G, Gohil K, Matsugo S, Moini H, Saliou C, Virgili F, Weber SU, Packer L (2001) Induction of glutathione synthesis in human keratinocytes by Ginkgo biloba extract (EGb761). BioFactors 15:39–52. https://doi.org/10.1002/biof.5520150104
Shi C, Fang L, Yew DT, Yao Z, Xu J (2010) Ginkgo biloba extract EGb761 protects against mitochondrial dysfunction in platelets and hippocampi in ovariectomized rats. Platelets 21:53–59. https://doi.org/10.3109/09537100903395180
Kaur S, Chhabra R, Nehru B (2013) Ginkgo biloba extract attenuates hippocampal neuronal loss and cognitive dysfunction resulting from trimethyltin in mice. Phytomedicine 20:178–186. https://doi.org/10.1016/j.phymed.2012.10.003
Gaucher C, Boudier A, Bonetti J, Clarot I, Leroy P, Parent M (2018) Glutathione: antioxidant properties dedicated to nanotechnologies. Antioxidants 7:1–21. https://doi.org/10.3390/antiox7050062
Cai W, Xue C, Sakaguchi M, Konishi M, Shirazian A, Ferris HA, Li ME, Yu R et al (2018) Insulin regulates astrocyte gliotransmission and modulates behavior. J Clin Invest 128:2914–2926. https://doi.org/10.1172/JCI99366
Carter AB, Hunninghake GW (2000) A constitutive active MEK → ERK pathway negatively regulates NF-κB-dependent gene expression by modulating TATA-binding protein phosphorylation. J Biol Chem 275:27858–27864. https://doi.org/10.1074/jbc.M003599200
Klinger MB, Sacks S, Cervero F (2011) A role for extracellular signal-regulated kinases 1 and 2 in the maintenance of persistent mechanical hyperalgesia in ovariectomized mice. Neuroscience 172:483–493. https://doi.org/10.1016/j.neuroscience.2010.10.043
Kim D Il, Choi MS, Pak SC, et al (2012) The effects of Sutaehwan-Gami on menopausal symptoms induced by ovariectomy in rats. BMC Complement Altern Med 12:1–8. https://doi.org/10.1186/1472-6882-12-227
Pereira RTS, Porto CS, Abdalla FMF (2014) Ovariectomy and 17β-estradiol replacement play a role on the expression of endonuclease-G and phosphorylated cyclic AMP response element-binding (CREB) protein in hippocampus. Mol Cell Endocrinol 382:227–233. https://doi.org/10.1016/j.mce.2013.09.037
Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y (2007) EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J 21:2400–2408. https://doi.org/10.1096/fj.06-7649com
Zhang W, Song JK, Yan R, Li L, Xiao ZY, Zhou WX, Wang ZZ, Xiao W et al (2018) Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol Sin 39:1259–1272. https://doi.org/10.1038/aps.2017.149
Acknowledgments
The authors gratefully acknowledge the support given by Suzete Maria Cerutti, Esther Milani Ático, Nelson Inácio Pinto Neto, Paloma Korehisa Maza, and Carol Anne Dannenhoffer.
Funding
This research was supported by grants from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) [2014/18929-9] and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) [finance code 001].
Author information
Authors and Affiliations
Contributions
Meira Maria Forcelini Machado—conceptualization, validation, formal analysis, investigation, and data curation
Renata Mancini Banin—conceptualization, methodology, visualization, and supervision
Fernanda Malanconi Thomaz—conceptualization, methodology, and investigation
Iracema Senna de Andrade—conceptualization, methodology, and investigation
Valter Tadeu Boldarine—conceptualization, methodology, and investigation
Jéssica de Souza Figueiredo—conceptualization, methodology, and investigation
Bruna Kelly Sousa Hirata—conceptualization, methodology, and data curation
Lila Missae Oyama—conceptualization, visualization, and funding acquisition
João Henrique Ghilardi Lago—conceptualization, visualization, methodology, formal analysis, resources, and writing—review and editing
Eliane Beraldi Ribeiro—conceptualization, visualization, and funding acquisition
Mônica Marques Telles—conceptualization, methodology, formal analysis, resources, writing—review and editing, visualization, supervision, project administration, and funding acquisition
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Machado, M.M.F., Banin, R.M., Thomaz, F.M. et al. Ginkgo biloba Extract (GbE) Restores Serotonin and Leptin Receptor Levels and Plays an Antioxidative Role in the Hippocampus of Ovariectomized Rats. Mol Neurobiol 58, 2692–2703 (2021). https://doi.org/10.1007/s12035-021-02281-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02281-5