Abstract
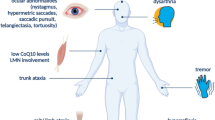
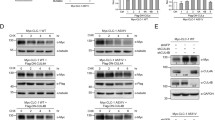
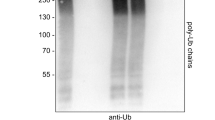
The 14 kDa histidine triad nucleotide-binding protein 1 (HINT1) is critical to maintain the normal function of motor neurons. Thus, a series of human HINT1 mutants cause autosomal recessive axonal neuropathy with neuromyotonia. HINT1 establishes a series of regulatory interactions with signaling proteins, some of which are enriched in motor neurons, such as the type 1 sigma receptor or intracellular domain (ICD) of transmembrane teneurin 1, both of which are also implicated in motor disturbances. In a previous study, we reported the capacity of HINT1 to remove the small ubiquitin-like modifier (SUMO) from a series of substrates and the influence of HINT1 mutants on this activity. We now report how human HINT1 mutations affect the interaction of HINT1 with the regulator of its SUMOylase activity, calcium-activated calmodulin, and its substrate SUMO. Moreover, HINT1 mutants exhibited anomalous interactions with G protein coupled receptors, such as the mu-opioid, and with glutamate N-methyl-D-aspartate receptors as well. Additionally, these HINT1 mutants showed impaired associations with transcriptional regulators such as the regulator of G protein signaling Z2 protein and the cleaved N-terminal ICD of teneurin 1. Thus, the altered enzymatic activity of human HINT1 mutants and their anomalous interactions with partner proteins may disrupt signaling pathways essential to the normal function of human motor neurons.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Brzoska PM, Chen H, Levin NA, Kuo WL, Collins C, Fu KK, Gray JW, Christman MF (1996) Cloning, mapping, and in vivo localization of a human member of the PKCI-1 protein family (PRKCNH1). Genomics 36:151–156. https://doi.org/10.1006/geno.1996.0435
Chou TF, Tikh IB, Horta BA, Ghosh B, De Alencastro RB, Wagner CR (2007) Engineered monomeric human histidine triad nucleotide-binding protein 1 hydrolyzes fluorogenic acyl-adenylate and lysyl-tRNA synthetase-generated lysyl-adenylate. J Biol Chem 282:15137–15147. https://doi.org/10.1074/jbc.M606972200
Brenner C (2002) Hint, Fhit, and GalT: function, structure, evolution, and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases. Biochemistry 41:9003–9014. https://doi.org/10.1021/bi025942q
Chou TF, Wagner CR (2007) Lysyl-tRNA synthetase-generated lysyl-adenylate is a substrate for histidine triad nucleotide binding proteins. J Biol Chem 282:4719–4727. https://doi.org/10.1074/jbc.M610530200
Rodríguez-Muñoz M, Garzón J (2013) Nitric oxide and zinc-mediated protein assemblies involved in mu opioid receptor signaling. Mol Neurobiol 48:769–782. https://doi.org/10.1007/s12035-013-8465-z
Rodríguez-Muñoz M, Torre-Madrid E, Sánchez-Blázquez P, Garzón J (2011) NO-released zinc supports the simultaneous binding of Raf-1 and PKCgamma cysteine-rich domains to HINT1 protein at the mu-opioid receptor. Antioxid Redox Signal 14:2413–2425. https://doi.org/10.1089/ars.2010.3511
Cortés-Montero E, Rodríguez-Muñoz M, Sánchez-Blázquez P, Garzón J (2019) The axonal motor neuropathy-related HINT1 protein is a zinc- and calmodulin-regulated cysteine SUMO protease. Antioxid Redox Signal 31:503–520. https://doi.org/10.1089/ars.2019.7724
Zimon M, Baets J, Almeida-Souza L, De VE, Nikodinovic J, Parman Y et al (2012) Loss-of-function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nat Genet 44:1080–1083. https://doi.org/10.1038/ng.2406
Boerkoel CF, Takashima H, Garcia CA, Olney RK, Johnson J, Berry K, Russo P, Kennedy S et al (2002) Charcot-Marie-tooth disease and related neuropathies: mutation distribution and genotype-phenotype correlation. Ann Neurol 51:190–201. https://doi.org/10.1002/ana.10089
Peeters K, Chamova T, Tournev I, Jordanova A (2017) Axonal neuropathy with neuromyotonia: there is a HINT. Brain 140:868–877. https://doi.org/10.1093/brain/aww301
Seburn KL, Morelli KH, Jordanova A, Burgess RW (2014) Lack of neuropathy-related phenotypes in hint1 knockout mice. J Neuropathol Exp Neurol 73:693–701. https://doi.org/10.1097/NEN.0000000000000085
Liu Q, Puche AC, Wang JB (2008) Distribution and expression of protein kinase C interactive protein (PKCI/HINT1) in mouse central nervous system (CNS). Neurochem Res 33:1263–1276. https://doi.org/10.1007/s11064-007-9578-4
McDonald JR, Groschel-Stewart U, Walsh MP (1987) Properties and distribution of the protein inhibitor (Mr 17,000) of protein kinase C. Biochem J 242:695–705. https://doi.org/10.1042/bj2420695
Garzón J, Rodríguez-Muñoz M, Vicente-Sánchez A, Bailón C, Martínez-Murillo R, Sánchez-Blázquez P (2011) RGSZ2 binds to the neural nitric oxide synthase PDZ domain to regulate mu-opioid receptor-mediated potentiation of the N-methyl-D-aspartate receptor-calmodulin-dependent protein kinase II pathway. Antioxid Redox Signal 15:873–887. https://doi.org/10.1089/ars.2010.3767
Ajit SK, Ramineni S, Edris W, Hunt RA, Hum WT, Hepler JR, Young KH (2007) RGSZ1 interacts with protein kinase C interacting protein PKCI-1 and modulates mu opioid receptor signaling. Cell Signal 19:723–730. https://doi.org/10.1016/j.cellsig.2006.09.008
Rodríguez-Muñoz M, Sánchez-Blázquez P, Herrero-Labrador R, Martínez-Murillo R, Merlos M, Vela JM, Garzón J (2015) The sigma1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control. Antioxid Redox Signal 22:799–818. https://doi.org/10.1089/ars.2014.5993
Pearson JD, DeWald DB, Mathews WR, Mozier NM, Zurcher-Neely HA, Heinrikson RL et al (1990) Amino acid sequence and characterization of a protein inhibitor of protein kinase C. J Biol Chem 265:4583–4591
Rodríguez-Muñoz M, Cortés-Montero E, Pozo-Rodrigalvarez A, Sánchez-Blázquez P, Garzón-Niño J (2015) The ON:OFF switch, sigma1R-HINT1 protein, controls GPCR-NMDA receptor cross-regulation: implications in neurological disorders. Oncotarget 6:35458-35477. https://doi.org/10.18632/oncotarget.6064
Bodle CR, Mackie DI, Roman DL (2013) RGS17: an emerging therapeutic target for lung and prostate cancers. Future Med Chem 5:995–1007. https://doi.org/10.4155/fmc.13.91
Hayes MP, Roman DL (2016) Regulator of G protein signaling 17 as a negative modulator of GPCR signaling in multiple human cancers. AAPS J 18:550–559. https://doi.org/10.1208/s12248-016-9894-1
Weiske J, Huber O (2005) The histidine triad protein Hint1 interacts with Pontin and Reptin and inhibits TCF-beta-catenin-mediated transcription. J Cell Sci 118:3117–3129. https://doi.org/10.1242/jcs.02437
Scholer J, Ferralli J, Thiry S, Chiquet-Ehrismann R (2015) The intracellular domain of teneurin-1 induces the activity of microphthalmia-associated transcription factor (MITF) by binding to transcriptional repressor HINT1. J Biol Chem 290:8154–8165. https://doi.org/10.1074/jbc.M114.615922
Huebner K, Saldivar JC, Sun J, Shibata H, Druck T (2011) Hits, Fhits and nits: beyond enzymatic function. Adv Enzym Regul 51:208–217. https://doi.org/10.1016/j.advenzreg.2010.09.003
Li H, Balajee AS, Su T, Cen B, Hei TK, Weinstein IB (2008) The HINT1 tumor suppressor regulates both gamma-H2AX and ATM in response to DNA damage. J Cell Biol 183:253–265. https://doi.org/10.1083/jcb.200711150
Weiske J, Huber O (2006) The histidine triad protein Hint1 triggers apoptosis independent of its enzymatic activity. J Biol Chem 281:27356–27366. https://doi.org/10.1074/jbc.M513452200
Li H, Zhang Y, Su T, Santella RM, Weinstein IB (2006) Hint1 is a haplo-insufficient tumor suppressor in mice. Oncogene 25:713–721. https://doi.org/10.1038/sj.onc.1209111
Su T, Suzui M, Wang L, Lin CS, Xing WQ, Weinstein IB (2003) Deletion of histidine triad nucleotide-binding protein 1/PKC-interacting protein in mice enhances cell growth and carcinogenesis. Proc Natl Acad Sci U S A 100:7824–7829. https://doi.org/10.1073/pnas.1332160100
Zhang YJ, Li H, Wu HC, Shen J, Wang L, Yu MW, Lee PH, Bernard Weinstein I et al (2009) Silencing of Hint1, a novel tumor suppressor gene, by promoter hypermethylation in hepatocellular carcinoma. Cancer Lett 275:277–284. https://doi.org/10.1016/j.canlet.2008.10.042
Rodríguez-Muñoz M, Bermudez D, Sánchez-Blázquez P, Garzón J (2007) Sumoylated RGS-Rz proteins act as scaffolds for Mu-opioid receptors and G-protein complexes in mouse brain. Neuropsychopharmacology 32:842–850. https://doi.org/10.1038/sj.npp.1301184
Eifler K, Vertegaal AC (2015) Mapping the SUMOylated landscape. FEBS J 282:3669–3680. https://doi.org/10.1111/febs.13378
Lima CD, Klein MG, Weinstein IB, Hendrickson WA (1996) Three-dimensional structure of human protein kinase C interacting protein 1, a member of the HIT family of proteins. Proc Natl Acad Sci U S A 93:5357–5362. https://doi.org/10.1073/pnas.93.11.5357
Shah RM, Maize KM, West HT, Strom AM, Finzel BC, Wagner CR (2018) Structure and functional characterization of human histidine triad nucleotide-binding protein 1 mutations associated with inherited axonal neuropathy with neuromyotonia. J Mol Biol 430:2709–2721. https://doi.org/10.1016/j.jmb.2018.05.028
Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M (2000) Calmodulin target database. J Struct Funct Genomics 1:8-14. 10.1023/a: 1011320027914
Zhao Q, Xie Y, Zheng Y, Jiang S, Liu W, Mu W, Liu Z, Zhao Y et al (2014) GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic Acids Res 42:W325–W330. https://doi.org/10.1093/nar/gku383
Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, Bailón C, Martín-Aznar B, Garzón J (2011) The histidine triad nucleotide-binding protein 1 supports mu-opioid receptor-glutamate NMDA receptor cross-regulation. Cell Mol Life Sci 68:2933–2949. https://doi.org/10.1007/s00018-010-0598-x
Cen B, Li H, Weinstein IB (2009) Histidine triad nucleotide-binding protein 1 up-regulates cellular levels of p27KIP1 by targeting ScfSKP2 ubiquitin ligase and Src. J Biol Chem 284:5265–5276. https://doi.org/10.1074/jbc.M804531200
Wang L, Li H, Zhang Y, Santella RM, Weinstein IB (2009) HINT1 inhibits beta-catenin/TCF4, USF2 and NFkappaB activity in human hepatoma cells. Int J Cancer 124:1526–1534. https://doi.org/10.1002/ijc.24072
Lee YN, Nechushtan H, Figov N, Razin E (2004) The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity 20:145–151. https://doi.org/10.1016/s1074-7613(04)00020-2
Lee YN, Razin E (2005) Nonconventional involvement of LysRS in the molecular mechanism of USF2 transcriptional activity in FcepsilonRI-activated mast cells. Mol Cell Biol 25:8904–8912. https://doi.org/10.1128/MCB.25.20.8904-8912.2005
Kumar V, Chichili VP, Tang X, Sivaraman J (2013) A novel trans conformation of ligand-free calmodulin. PLoS One 8:e54834. https://doi.org/10.1371/journal.pone.0054834
Warren JT, Guo Q, Tang WJ (2007) A 1.3-A structure of zinc-bound N-terminal domain of calmodulin elucidates potential early ion-binding step. J Mol Biol 374:517–527. https://doi.org/10.1016/j.jmb.2007.09.048
Guang W, Wang H, Su T, Weinstein IB, Wang JB (2004) Role of mPKCI, a novel mu-opioid receptor interactive protein, in receptor desensitization, phosphorylation, and morphine-induced analgesia. Mol Pharmacol 66:1285–1292. https://doi.org/10.1124/mol.66.5
Rodríguez-Muñoz M, Torre-Madrid E, Sánchez-Blázquez P, Wang JB, Garzón J (2008) NMDAR-nNOS generated zinc recruits PKCgamma to the HINT1-RGS17 complex bound to the C terminus of Mu-opioid receptors. Cell Signal 20:1855–1864. https://doi.org/10.1016/j.cellsig.2008.06.015
Ehlers MD, Zhang S, Bernhadt JP, Huganir RL (1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84:745–755. https://doi.org/10.1016/s0092-8674(00)81052-1
Lipton SA (2006) Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov 5:160–170. https://doi.org/10.1038/nrd1958
Lu H, Le WD, Xie YY, Wang XP (2016) Current therapy of drugs in amyotrophic lateral sclerosis. Curr Neuropharmacol 14:314–321. https://doi.org/10.2174/1570159x14666160120152423
Bastias-Candia S, Braidy N, Zolezzi JM, Inestrosa NC (2015) Teneurins and Alzheimer's disease: a suggestive role for a unique family of proteins. Med Hypotheses 84:402–407. https://doi.org/10.1016/j.mehy.2015.01.026
Leamey CA, Sawatari A (2019) Teneurins: mediators of complex neural circuit assembly in mammals. Front Neurosci 13:580. https://doi.org/10.3389/fnins.2019.00580
Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L (2012) Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature 484:237–241. https://doi.org/10.1038/nature10923
Hor H, Francescatto L, Bartesaghi L, Ortega-Cubero S, Kousi M, Lorenzo-Betancor O, Jiménez-Jiménez FJ, Gironell A et al (2015) Missense mutations in TENM4, a regulator of axon guidance and central myelination, cause essential tremor. Hum Mol Genet 24:5677–5686. https://doi.org/10.1093/hmg/ddv281
Suzuki N, Fukushi M, Kosaki K, Doyle AD, de Vega S, Yoshizaki K et al (2012) Teneurin-4 is a novel regulator of oligodendrocyte differentiation and myelination of small-diameter axons in the CNS. J Neurosci 32:11586–11599. https://doi.org/10.1523/JNEUROSCI.2045-11.2012
McMichael G, Bainbridge MN, Haan E, Corbett M, Gardner A, Thompson S, van Bon BWM, van Eyk CL et al (2015) Whole-exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry 20:176–182. https://doi.org/10.1038/mp.2014.189
Garzón J, Rodríguez-Muñoz M, Vicente-Sánchez A, García-López MA, Martínez-Murillo R, Fischer T, Sánchez-Blázquez P (2011) SUMO-SIM interactions regulate the activity of RGSZ2 proteins. PLoS One 6:e28557. https://doi.org/10.1371/journal.pone.0028557
Hayashi T, Su TP (2003) Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306:718–725. https://doi.org/10.1124/jpet.103.051284
Sánchez-Blázquez P, Rodríguez-Muñoz M, Herrero-Labrador R, Burgueño J, Zamanillo D, Garzón J (2014) The calcium-sensitive Sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: implications in antinociception and psychotic diseases. Int J Neuropsychopharmacol 17:1943–1955. https://doi.org/10.1017/S1461145714000029
Kim FJ, Kovalyshyn I, Burgman M, Neilan C, Chien CC, Pasternak GW (2010) Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Pharmacol 77:695–703. https://doi.org/10.1124/mol.109.057083
Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J et al (2010) Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A 107:18676–18681. https://doi.org/10.1073/pnas.1008911107
Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE (2012) The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol 682:12–20. https://doi.org/10.1016/j.ejphar.2012.01.030
Tsai SY, Chuang JY, Tsai MS, Wang XF, Xi ZX, Hung JJ et al (2015) Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc Natl Acad Sci U S A 112:E6562–E6570. https://doi.org/10.1073/pnas.1518894112
Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE (2010) The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience 167:247–255. https://doi.org/10.1016/j.neuroscience.2010.02.022
Gundlach AL, Largent BL, Snyder SH (1986) Autoradiographic localization of sigma receptor binding sites in Guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci 6:1757–1770
Gregianin E, Pallafacchina G, Zanin S, Crippa V, Rusmini P, Poletti A, Fang M, Li Z et al (2016) Loss-of-function mutations in the SIGMAR1 gene cause distal hereditary motor neuropathy by impairing ER-mitochondria tethering and Ca2+ signalling. Hum Mol Genet 25:3741–3753. https://doi.org/10.1093/hmg/ddw220
Almendra L, Laranjeira F, Fernandez-Marmiesse A, Negrao L (2018) SIGMAR1 gene mutation causing distal hereditary motor neuropathy in a Portuguese family. Acta Myol 37:2–4
Luty AA, Kwok JB, Dobson-Stone C, Loy CT, Coupland KG, Karlstrom H et al (2010) Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann Neurol 68:639–649. https://doi.org/10.1002/ana.22274
Ullah MI, Ahmad A, Raza SI, Amar A, Ali A, Bhatti A, John P, Mohyuddin A et al (2015) In silico analysis of SIGMAR1 variant (rs4879809) segregating in a consanguineous Pakistani family showing amyotrophic lateral sclerosis without frontotemporal lobar dementia. Neurogenetics 16:299–306. https://doi.org/10.1007/s10048-015-0453-1
Watanabe S, Ilieva H, Tamada H, Nomura H, Komine O, Endo F et al. (2016) Mitochondria-associated membrane collapse is a common pathomechanism in SIG. EMBO Mol Med 8:1421-1437. https://doi.org/10.15252/emmm.201606403
Al-Saif A, Al-Mohanna F, Bohlega S (2011) A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol 70:913–919. https://doi.org/10.1002/ana.22534
Rodríguez-Muñoz M, Cortés-Montero E, Garzón-Niño J, Sánchez-Blázquez P (2020) The ALS-related sigma1R E102Q mutant eludes ligand control and exhibits anomalous response to calcium. Int J Mol Sci 21 https://doi.org/10.3390/ijms21197339
Dreser A, Vollrath JT, Sechi A, Johann S, Roos A, Yamoah A, Katona I, Bohlega S et al (2017) The ALS-linked E102Q mutation in sigma receptor-1 leads to ER stress-mediated defects in protein homeostasis and dysregulation of RNA-binding proteins. Cell Death Differ 24:1655–1671. https://doi.org/10.1038/cdd.2017.88
Bernard-Marissal N, Medard JJ, Azzedine H, Chrast R (2015) Dysfunction in endoplasmic reticulum-mitochondria crosstalk underlies SIGMAR1 loss of function mediated motor neuron degeneration. Brain 138:875–890. https://doi.org/10.1093/brain/awv008
Shah RM, Peterson C, Strom A, Dillenburg M, Finzel B, Kitto KF, Fairbanks C, Wilcox G et al (2019) Inhibition of HINT1 modulates spinal nociception and NMDA evoked behavior in mice. ACS Chem Neurosci 10:4385–4393. https://doi.org/10.1021/acschemneuro.9b00432
Garzón J, Herrero-Labrador R, Rodríguez-Muñoz M, Shah R, Vicente-Sánchez A, Wagner CR, Sánchez-Blázquez P (2015) HINT1 protein: a new therapeutic target to enhance opioid antinociception and block mechanical allodynia. Neuropharmacology 89:412–423. https://doi.org/10.1016/j.neuropharm.2014.10.022
Acknowledgments
Elsa Cortés-Montero is a recipient of a Fellowship from MECD [FPU 15/02356]. We would like to thank Gabriela de Alba and María José López for their excellent technical assistance. We also appreciate the advice of the Department of Statistics, Center for Informatics at the Spanish Council for Scientific Research (CSIC).
Funding
This work was supported by Plan Nacional I+D+i [grant number RT-2018-093677-B-100].
Author information
Authors and Affiliations
Contributions
The JGN, ECM, and MRM designed the research. ECM and JGN wrote the manuscript. JGN and PSB obtained the funding. ECM and MRM performed the experiments and the statistical analysis of data. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures were approved by the Spanish government and in accordance with the guidelines of the European Communities Council Directives.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2875 kb)
Rights and permissions
About this article
Cite this article
Cortés-Montero, E., Rodríguez-Muñoz, M., Sánchez-Blázquez, P. et al. Human HINT1 Mutant Proteins that Cause Axonal Motor Neuropathy Exhibit Anomalous Interactions with Partner Proteins. Mol Neurobiol 58, 1834–1845 (2021). https://doi.org/10.1007/s12035-020-02265-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02265-x