Abstract
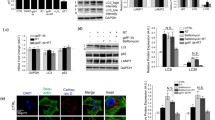
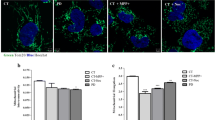
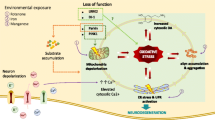
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra (SN) pars compacta region of the brain. The main pathological hallmark involves cytoplasmic inclusions of α-synuclein and mitochondrial dysfunction, which is observed in other part of the central nervous system other than SN suggesting the spread of pathogenesis to bystander neurons. The inter-neuronal communication through exosomes may play an important role in the spread of the disease; however, the mechanisms are not well elucidated. Mitochondria and its role in inter-organellar crosstalk with multivesicular body (MVB) and lysosome and its role in modulation of exosome release in PD is not well understood. In the current study, we investigated the mitochondria-lysosome crosstalk modulating the exosome release in neuronal and glial cells. We observed that PD stress showed enhanced release of exosomes in dopaminergic neurons and glial cells. The PD stress condition in these cells showed fragmented network and mitochondrial dysfunction which further leads to functional deficit of lysosomes and hence inhibition of autophagy flux. Neuronal and glial cells treated with rapamycin showed enhanced autophagy and inhibited the exosomal release. The results here suggest that maintenance of mitochondrial function is important for the lysosomal function and hence exosomal release which is important for the pathogenesis of PD.





Similar content being viewed by others
References
Draoui A, El Hiba O, Aimrane A et al (2020) General review Parkinson’s disease: from bench to bedside. Rev Neurol (Paris) 176:1–17. https://doi.org/10.1016/j.neurol.2019.11.002
Jellinger KA (2014) Neuropathology of Parkinson’ s disease. Springer, Inflammation in Parkinson's Disease, pp. 25–74. https://doi.org/10.1007/978-3-319-08046-8
Beyer K, Ariza A (2009) Molecular pathology of Lewy body diseases. Int J Mol Sci:724–745. https://doi.org/10.3390/ijms10030724
Henderson MX, Cornblath EJ, Darwich A, Zhang B, Brown H, Gathagan RJ, Sandler RM, Bassett DS et al (2019) Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat Neurosci 22:1248–1257. https://doi.org/10.1038/s41593-019-0457-5
Lill CM (2016) Genetics of Parkinson’s disease. Mol Cell Probes 30:386–396. https://doi.org/10.1016/j.mcp.2016.11.001
Braak RH, Ghebremedhin E, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res:121–134. https://doi.org/10.1007/s00441-004-0956-9
Frühbeis C, Fröhlich D, Kuo WP (2013) Extracellular vesicles as mediators of neuron – glia communication. Front Cell Neurosci 7:1–6. https://doi.org/10.3389/fncel.2013.00182
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. https://doi.org/10.1083/jcb.201211138
Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75:193–208. https://doi.org/10.1007/s00018-017-2595-9
Desplats P, Lee H, Bae E et al (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α -synuclein. PNAS 106(31):13010–13015. https://doi.org/10.1073/pnas.0903691106
Lee H, Suk J, Patrick C et al (2010) Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285:9262–9272. https://doi.org/10.1074/jbc.M109.081125
Perier C, Vila M (2012) Mitochondrial biology and Parkinson’s disease. Cold Spring Harbor Perspectives in medicine 2:1–19. https://doi.org/10.1101/cshperspect.a009332
Vries D, Rosa LA, Przedborski S (2013) Mitophagy and Parkinson’s disease: be eaten to stay healthy. Mol Cell Neurosci 55:37–43. https://doi.org/10.1016/j.mcn.2012.07.008
Narendra D, Tanaka A, Suen D, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803. https://doi.org/10.1083/jcb.200809125
Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S et al (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189:211–221. https://doi.org/10.1083/jcb.200910140
He X, Yuan W, Li Z, Hou Y, Liu F, Feng J (2018) 6-Hydroxydopamine induces autophagic flux dysfunction by impairing transcription factor EB activation and lysosomal function in dopaminergic neurons and SH-SY5Y cells. Toxicol Lett 283:58–68. https://doi.org/10.1016/j.toxlet.2017.11.017
Ramirez A, Gru J, Stiller B et al (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2 , encoding a lysosomal type 5 ATPase. 38:1184–1191. https://doi.org/10.1038/ng1884
Dehay B, Ramirez A, Martinez-vicente M et al (2012) Loss of P-type ATPase ATP13A2 / PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. PNAS 109(24):9611–9616. https://doi.org/10.1073/pnas.1112368109
Richaud-patin Y, Carballo-carbajal I, Sa A et al (2012) Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson ’ s disease. EMBO Mol Med:380–395. https://doi.org/10.1002/emmm.201200215
Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B et al (2011) LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8:267–280. https://doi.org/10.1016/j.stem.2011.01.013
Baixauli F, López-otín C, Mittelbrunn M (2014) Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 5:1–6. https://doi.org/10.3389/fimmu.2014.00403
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30:3481–3500. https://doi.org/10.1038/emboj.2011.286
Colombo M (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol:255–292. https://doi.org/10.1146/annurev-cellbio-101512-122326
Plotegher N, Duchen MR (2017) Crosstalk between lysosomes and mitochondria in Parkinson’s disease. Frontiers in cell and developmental biology 5:2011–2018. https://doi.org/10.3389/fcell.2017.00110
Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H et al (2018) Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20:332–343. https://doi.org/10.1038/s41556-018-0040-4
Galindo F, Tornero D, Gonza C (2004) Bcl-x L blocks mitochondrial multiple conductance channel activation and inhibits 6-OHDA-induced death in SH-SY5Y cells. J Neurochem:124–133. https://doi.org/10.1046/j.1471-4159.2003.02299.x
Xiong N, Long X, Xiong J et al (2012) Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson ’ s disease models. Crit Rev Toxicol:1–20. https://doi.org/10.3109/10408444.2012.680431
Savina A, Vidal M, Colombo MI (2002) The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci 115(12):2505–2515
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525. https://doi.org/10.1074/jbc.M210432200
Baixauli F, Acín-pérez R, Villarroya-beltrí C et al (2015) Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab 22:485–498. https://doi.org/10.1016/j.cmet.2015.07.020
Demers-lamarche J, Guillebaud G, Tlili M et al (2016) Loss of mitochondrial function impairs lysosomes. J Biol Chem 291:10263–10276. https://doi.org/10.1074/jbc.M115.695825
Cortes CJ, La Spada AR, Biology C (2019) TFEB dysregulation as a driver of autophagy dysfunction in neurodegenerative disease: Molecular mechanisms, cellular processes, and emerging therapeutic opportunities. Neurobiol Dis 122:83–93. https://doi.org/10.1016/j.nbd.2018.05.012
Cheung ZH, Ip NY (2009) The emerging role of autophagy in Parkinson’s disease. Molecular Brain 6:1–6. https://doi.org/10.1186/1756-6606-2-29
Kimura S, TN& TY (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3, Autophagy, 452–460. https://doi.org/10.4161/auto.4451
Chittaranjan S, Bortnik S, Gorski SM (2015) Monitoring autophagic flux by using lysosomal inhibitors and western blotting of endogenous MAP1LC3B:743–751. https://doi.org/10.1101/pdb.prot086256
Xu J, Camfield R, Gorski SM (2018) The interplay between exosomes and autophagy – partners in crime. J Cell Sci 131:1–11. https://doi.org/10.1242/jcs.215210
Malagelada C, Jin ZH, Jackson-lewis V et al (2010) Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson ’ s disease. J Neurosci 30:1166–1175. https://doi.org/10.1523/JNEUROSCI.3944-09.2010
Alexander GE (2004) Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci:259–280
Langston JW, Forno LS, Tetrud J, Reeves AG (1999) Evidence of active nerve cell degeneration in the Substantia Nigra of humans years after 1-methyl-4-phenyl, 1,2,3,6- tetrahydropyridine exposure. Annals of Neurology: Official Journal of the american neurological Association and the child Neurology Society:598–605
Bronstein DM, Sun V, Sawin SBM et al (1995) Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res 704:112–116
Kim W, Mohney RP, Wilson B et al (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain : role of microglia. J Neurosci 20:6309–6316
Schumacker S (2013) Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem 288:10736–10741. https://doi.org/10.1074/jbc.R112.410530
Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J et al (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α -Synuclein. J Biol Chem 286:20710–20726. https://doi.org/10.1074/jbc.M110.213538
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of α -Synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283:9089–9100. https://doi.org/10.1074/jbc.M710012200
Parker J, Parks KRHS (2008) Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res:215–218
Keeney PM, Xie J, Capaldi RA, Bennett JP Jr (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26:5256–5264. https://doi.org/10.1523/JNEUROSCI.0984-06.2006
Chan P, Delanney LE, Irwin I et al (1991) Rapid ATP loss caused by 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine in mouse brain. J Neurochem:348–351. https://doi.org/10.1111/j.1471-4159.1991.tb02134.x
Tretter L, Sipos I, Adam-vizi V (2004) Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson’s disease. Neurochem Res 29:569–577
Parker WD, Boyson TSJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Annals of Neurology: Official Journal of the american neurological Association and the child Neurology Society 26(6):719–723
Schapira A, H. V, et al (1990) Mitochondria1 complex I deficiency in Parkinson’s disease, J Neurochem, 54(3):823–827
Polito L, Greco A, Seripa D (2016) Genetic profile, environmental exposure, and their interaction in Parkinson’s disease. Parkinson's Disease. https://doi.org/10.1155/2016/6465793
Caspersen C, Jackson-lewis V, Carelli V et al (2005) Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. PNAS 102(52):19126–19131. https://doi.org/10.1073/pnas.0508215102
Ramachandran PV, Savini M, Folick AK, Hu K (2019) Lysosomal signaling promotes longevity through adjusting mitochondrial activity. Dev Cell 48:685–696. https://doi.org/10.1016/j.devcel.2018.12.022
Martini-stoica H, Xu Y, Ballabio A et al (2017) The autophagy–lysosomal pathway in neurodegeneration: A TFEB perspective. Trends Neurosci 39:221–234. https://doi.org/10.1016/j.tins.2016.02.002
Jaber N, Mohd-naim N, Wang Z et al (2016) Vps34 regulates Rab7 and late endocytic trafficking through recruitment of the GTPase activating protein Armus. J Cell Sci 129(23):4424–4435. https://doi.org/10.1242/jcs.192260
Acknowledgements
Authors acknowledge the DBT-MSUB-ILSPARE programme of the Department of Biochemistry, the M S University of Baroda sponsored by DBT, Government of India. Authors also acknowledge the FIST programme supported by DST, Government of India. This work is a part of the Ph.D. thesis of Fatema Currim. Fatema Currim received Junior Research Fellowship from DST-INSPIRE, Government of India. Dhruv Gohel received Senior Research Fellowship from the Indian Council of Medical Research (ICMR), Government of India. Minal Mane received Senior Research fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India.
Funding
The current research work was financially supported by the Department of Biotechnology, Government of India grant (BT/PR19937/MED/122/17/2016) to RS.
Author information
Authors and Affiliations
Contributions
Fatema Currim: Investigation, validation, writing—original draft
Jyoti Singh Anjali Shinde, Milton Roy, Dhruv Gohel, Kritarth Singh, Shatakshi Shukla, Minal Mane and Hitesh Vasiyani: Formal data analysis, methodology and resources
Rajesh Singh: Conceptualization, methodology, resources, writing—original draft, visualization, supervision and funding acquisition
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 541 kb).
Rights and permissions
About this article
Cite this article
Currim, F., Singh, J., Shinde, A. et al. Exosome Release Is Modulated by the Mitochondrial-Lysosomal Crosstalk in Parkinson’s Disease Stress Conditions. Mol Neurobiol 58, 1819–1833 (2021). https://doi.org/10.1007/s12035-020-02243-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02243-3