Abstract
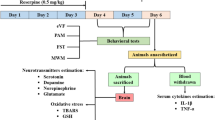
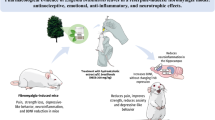
Fibromyalgia (FM) is one of the most common musculoskeletal pain conditions. Although the aetiology of FM is still unknown, mitochondrial dysfunction and the overproduction of reactive oxygen intermediates (ROI) are common characteristics in its pathogenesis. The reserpine experimental model can induce FM-related symptoms in rodents by depleting biogenic amines. However, it is unclear whether reserpine causes other pathophysiologic characteristics of FM. So far, no one has investigated the relevance of mitochondrial dysfunction in the reserpine-induced experimental FM model using protection- and insult-based mitochondrial modulators. Reserpine (1 mg/kg) was subcutaneously injected once daily for three consecutive days in male Swiss mice. We carried out analyses of reserpine-induced FM-related symptoms, and their modulation by using mitochondrial insult on ATP synthesis (oligomycin; 1 mg/kg, intraperitoneally) or mitochondrial protection (coenzyme Q10; 150 mg/kg/5 days, orally). We also evaluated the effect of reserpine on mitochondrial function using high-resolution respirometry and oxidative status. Reserpine caused nociception, loss in muscle strength, and anxiety- and depressive-like behaviours in mice that were consistent with clinical symptoms of FM, without inducing body weight and temperature alterations or motor impairment. Reserpine-induced FM-related symptoms were increased by oligomycin and reduced by coenzyme Q10 treatment. Reserpine caused mitochondrial dysfunction by negatively modulating the electron transport system and mitochondrial respiration (ATP synthesis) mainly in oxidative muscles and the spinal cord. These results support the role of mitochondria in mediating oxidative stress and FM symptoms in this model. In this way, reserpine-inducing mitochondrial dysfunction and increased production of ROI contribute to the development and maintenance of nociceptive, fatigue, and depressive-like behaviours.






Similar content being viewed by others
Abbreviations
- FM:
-
Fibromyalgia
- ROI:
-
Reactive oxygen intermediates
- CoQ10:
-
Coenzyme Q10
- H2O2 :
-
Hydrogen peroxide
- PWT:
-
Paw withdrawal threshold
- B1:
-
Baseline 1
- B2:
-
Baseline 2
- BIOPS:
-
Biopsy preservation solution
- MIR05:
-
Mitochondrial respiration medium 05
- HRR:
-
High-resolution respirometry
- SUIT:
-
Titration of multiple substrates, uncouplers, and inhibitors
- CI:
-
Complex I
- CII:
-
Complex II
- OXPHOS:
-
Oxidative phosphorylation
- PMG:
-
Pyruvate, malate, and glutamate
- ETS:
-
Electron transport system
- CS:
-
Citrate synthase
- LDH:
-
Lactate dehydrogenase
- DCF:
-
Dichlorofluorescein
- DCFDA:
-
Dichlorofluorescein diacetate
- HRPO:
-
Horseradish peroxidase
- CAT:
-
Catalase
- TBARS:
-
Thiobarbituric acid reactive species
References
Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S et al (2015) A classification of chronic pain for ICD-11. Pain 156:1. https://doi.org/10.1097/j.pain.0000000000000160
Clauw DJ (2014) Fibromyalgia: a clinical review. JAMA 311:1547–1555. https://doi.org/10.1001/jama.2014.3266
Choy EHS (2015) The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol 11:513–520. https://doi.org/10.1038/nrrheum.2015.56
Larson AA, Pardo JV, Pasley JD (2014) Review of overlap between thermoregulation and pain modulation in fibromyalgia. Clin J Pain 30:544–555. https://doi.org/10.1097/AJP.0b013e3182a0e383
Nishiyori M, Ueda H (2008) Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol Pain 4:475–476. https://doi.org/10.1002/acr
Cordero MD, De Miguel M, Moreno Fernández AM et al (2010) Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease. Arthritis Res Ther 12:R17. https://doi.org/10.1186/ar2918
Sánchez-Domínguez B, Bullón P, Román-Malo L, Marín-Aguilar F, Alcocer-Gómez E, Carrión AM, Sánchez-Alcazar JA, Cordero MD (2015) Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with fibromyalgia. Mitochondrion 21:69–75. https://doi.org/10.1016/j.mito.2015.01.010
Meeus M, Nijs J, Hermans L, Goubert D, Calders P (2013) The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: peripheral and central mechanisms as therapeutic targets? Expert Opin Ther Targets 17:1081–1089. https://doi.org/10.1517/14728222.2013.818657
Cordero MD, Cotán D, del Pozo Martín Y et al (2012) Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition 28:1200–1203. https://doi.org/10.1016/j.nut.2012.03.018
Cordero MD, Cano-García FJ, Alcocer-Gómez E, de Miguel M, Sánchez-Alcázar JA (2012) Oxidative stress correlates with headache symptoms in fibromyalgia: coenzyme Q 10 effect on clinical improvement. PLoS One 7:6–11. https://doi.org/10.1371/journal.pone.0035677
Alcocer-Gómez E, Culic O, Navarro-Pando JM, Sánchez-Alcázar JA, Bullón P (2017) Effect of coenzyme Q10 on psychopathological symptoms in fibromyalgia patients. CNS Neurosci Ther 23:188–189. https://doi.org/10.1111/cns.12668
Taguchi T, Katanosaka K, Yasui M, Hayashi K, Yamashita M, Wakatsuki K, Kiyama H, Yamanaka A et al (2015) Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain 156:415–427. https://doi.org/10.1097/01.j.pain.0000460334.49525.5e
Nagakura Y, Oe T, Aoki T, Matsuoka N (2009) Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: a putative animal model of fibromyalgia. Pain 146:26–33. https://doi.org/10.1016/j.pain.2009.05.024
Klein CP, Sperotto NDM, Maciel IS, Leite CE, Souza AH, Campos MM (2014) Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology 86:57–66. https://doi.org/10.1016/j.neuropharm.2014.05.043
Oezel L, Then H, Jung AL, Jabari S, Bonaterra GA, Wissniowski TT, Önel SF, Ocker M et al (2016) Fibromyalgia syndrome: metabolic and autophagic processes in intermittent cold stress mice. Pharmacol Res Perspect 4:1–13. https://doi.org/10.1002/prp2.248
Bonaterra GA, Then H, Oezel L, Schwarzbach H, Ocker M, Thieme K, di Fazio P, Kinscherf R (2016) Morphological alterations in gastrocnemius and soleus muscles in male and female mice in a fibromyalgia model. PLoS One 11:e0151116. https://doi.org/10.1371/journal.pone.0151116
Yüksel E, Nazlroǧlu M, Şahin M, Çiǧ B (2017) Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: protective role of selenium. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-17715-1
Favero G, Bonomini F, Franco C, Rezzani R (2019) Mitochondrial dysfunction in skeletal muscle of a fibromyalgia model: the potential benefits of melatonin. Int J Mol Sci 20:765. https://doi.org/10.3390/ijms20030765
Shukla VH, Dave KR, Katyare SS (2000) Effect of catecholamine depletion on oxidative energy metabolism in rat liver, brain and heart mitochondria; use of reserpine. Comp Biochem Physiol C Toxicol Pharmacol 127:79–90. https://doi.org/10.1016/S0742-8413(00)00134-1
Weinbach EC, Costa JL, Claggett CE, Fay DD, Hundal T (1983) Reserpine as an uncoupler of oxidative phosphorylation and the relevance to its psychoactive properties. Biochem Pharmacol 32:1371–1377
Brasil M das CT e I (2018) RESOLUÇÃO NORMATIVA No 39, DE 20 DE JUNHO DE 2018. Diário Oficial da União 120:7
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110
NIH NI of H guide for the care and use of L animals (2011) Guide for the Care and Use of Laboratory Animals, 8th ed
McGrath JC, Lilley E (2015) Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172:3189–3193. https://doi.org/10.1111/bph.12955
Brusco I, Justino AB, Silva CR, Fischer S, Cunha TM, Scussel R, Machado-de-Ávila RA, Ferreira J et al (2019) Kinins and their B1 and B2 receptors are involved in fibromyalgia-like pain symptoms in mice. Biochem Pharmacol 168:119–132. https://doi.org/10.1016/j.bcp.2019.06.023
Dagnino APA, Silva RBM, Chagastelles PC et al (2019) Nociceptin/orphanin FQ receptor modulates painful and fatigue symptoms in a mouse model of fibromyalgia. Pain 00:1383–1401. https://doi.org/10.1097/j.pain.0000000000001513
De Benedetto GE, Fico D, Pennetta A et al (2014) A rapid and simple method for the determination of 3,4-dihydroxyphenylacetic acid, norepinephrine, dopamine, and serotonin in mouse brain homogenate by HPLC with fluorimetric detection. J Pharm Biomed Anal 98:266–270. https://doi.org/10.1016/j.jpba.2014.05.039
da Silva Brum E, da Rosa Moreira L, da Silva ARH et al (2016) Tabernaemontana catharinensis ethyl acetate fraction presents antinociceptive activity without causing toxicological effects in mice. J Ethnopharmacol 191:115–124. https://doi.org/10.1016/j.jep.2016.06.036
Atwal N, Casey SL, Mitchell VA, Vaughan CW (2019) THC and gabapentin interactions in a mouse neuropathic pain model. Neuropharmacology 144:115–121. https://doi.org/10.1016/j.neuropharm.2018.10.006
Oliveira SM, Silva CR, Ferreira J (2013) Critical role of protease-activated receptor 2 activation by mast cell tryptase in the development of postoperative pain. Anesthesiology 118:679–690
Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462
Luna-sánchez M, Díaz-casado E, Barca E et al (2015) The clinical heterogeneity of coenzyme Q 10 deficiency results from genotypic differences in the Coq 9 gene. EMBO Mol Med 7:670–687
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Xiao WH, Bennett GJ (2012) Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 153:704–709. https://doi.org/10.1016/j.pain.2011.12.011
Zhang YP, Eber A, Yuan Y, Yang Z, Rodriguez Y, Levitt RC, Takacs P, Candiotti KA (2013) Prophylactic and antinociceptive effects of coenzyme Q10 on diabetic neuropathic pain in a mouse model of type 1 diabetes. Anesthesiology 118:945–954
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Martins EL, Ricardo JC, de Souza-Ferreira E et al (2018) Rapid regulation of substrate use for oxidative phosphorylation during a single session of high intensity interval or aerobic exercises in different rat skeletal muscles. Comp Biochem Physiol B Biochem Mol Biol 217:40–50. https://doi.org/10.1016/j.cbpb.2017.11.013
Gonçalves DF, Courtes AA, Hartmann DD, Pamela C (2019) 6-Hydroxydopamine induces different mitochondrial bioenergetics response in brain regions of rat. Neurotoxicology 70:1–11. https://doi.org/10.1016/j.neuro.2018.10.005
Zheng H, Xiao WH, Bennett GJ (2011) Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol 232:154–161. https://doi.org/10.1016/j.expneurol.2011.08.016
Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS (2008) Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3:965–976. https://doi.org/10.1038/nprot.2008.61
Eigentler A, Draxl A, Wiethüchter A et al (2015) Laboratory protocol : citrate synthase a mitochondrial marker enzyme. Mitochondrial Physiol Network 04:1–11
da Silva Brum E, Becker G, Fialho MFP et al (2019) TRPA1 involvement in analgesia induced by Tabernaemontana catharinensis ethyl acetate fraction in mice. Phytomedicine 54:248–258. https://doi.org/10.1016/j.phymed.2018.09.201
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Pegoraro NS, Barbieri AV, Camponogara C, Mattiazzi J, Brum ES, Marchiori MCL, Oliveira SM, Cruz L (2017) Nanoencapsulation of coenzyme Q10 and vitamin E acetate protects against UVB radiation-induced skin injury in mice. Colloids Surf B: Biointerfaces 150:32–40. https://doi.org/10.1016/j.colsurfb.2016.11.013
Clauw DJ (2015) Fibromyalgia and related conditions. Mayo Clin Proc 90:680–692. https://doi.org/10.1016/j.mayocp.2015.03.014
Berglund B, Harju EL, Kosek E, Lindblom U (2002) Quantitative and qualitative perceptual analysis of cold dysesthesia and hyperalgesia in fibromyalgia. Pain 96:177–187. https://doi.org/10.1016/S0304-3959(01)00443-2
Li S, Han J, Wang D, Feng B, Deng YT, Wang XS, Yang Q, Zhao MG (2016) Echinocystic acid reduces reserpine-induced pain/depression dyad in mice. Metab Brain Dis 31:455–463. https://doi.org/10.1007/s11011-015-9786-6
Fischer SPM, Brusco I, Brum ES, Fialho MFP, Camponogara C, Scussel R, Machado-de-Ávila RA, Trevisan G et al (2020) Involvement of TRPV1 and the efficacy of α-spinasterol on experimental fibromyalgia symptoms in mice. Neurochem Int 134:104673. https://doi.org/10.1016/j.neuint.2020.104673
Chinn S, Caldwell W, Gritsenko K (2016) Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep 20:1–10. https://doi.org/10.1007/s11916-016-0556-x
Liu SB, Zhao R, Li XS, Guo HJ, Tian Z, Zhang N, Gao GD, Zhao MG (2014) Attenuation of reserpine-induced pain/depression dyad by Gentiopicroside through downregulation of GluN2B receptors in the amygdala of mice. NeuroMolecular Med 16:350–359. https://doi.org/10.1007/s12017-013-8280-8
Nagakura Y, Ohsaka N, Azuma R, Takahashi S, Takebayashi Y, Kawasaki S, Murai S, Miwa M et al (2018) Monoamine system disruption induces functional somatic syndromes associated symptomatology in mice. Physiol Behav 194:505–514. https://doi.org/10.1016/j.physbeh.2018.07.007
Cordero MD, Moreno-Fernández AM, deMiguel M, Bonal P, Campa F, Jiménez-Jiménez LM, Ruiz-Losada A, Sánchez-Domínguez B et al (2009) Coenzyme Q10 distribution in blood is altered in patients with fibromyalgia. Clin Biochem 42:732–735. https://doi.org/10.1016/j.clinbiochem.2008.12.010
Pieczenik SR, Neustadt J (2007) Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 83:84–92. https://doi.org/10.1016/j.yexmp.2006.09.008
Salehpour F, Farajdokht F, Cassano P, Sadigh-Eteghad S, Erfani M, Hamblin MR, Salimi MM, Karimi P et al (2019) Near-infrared photobiomodulation combined with coenzyme Q10 for depression in a mouse model of restraint stress: reduction in oxidative stress, neuroinflammation, and apoptosis. Brain Res Bull 144:213–222. https://doi.org/10.1016/j.brainresbull.2018.10.010
Castro-Marrero J, Cordero MD, Sáez-Francas N, Jimenez-Gutierrez C, Aguilar-Montilla FJ, Aliste L, Alegre-Martin J (2013) Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal 19:1855–1860. https://doi.org/10.1089/ars.2013.5346
Espejo-Antúnez L, Cardero-Durán MA, Garrido-Ardila EM et al (2013) Clinical effectiveness of mud pack therapy in knee osteoarthritis. Rheumatology (Oxford, England) 52:659–668. https://doi.org/10.1093/rheumatology/kes322
Cordero MD, Moreno-Fernández AM, Carmona-López MI, Sánchez-Alcázar JA, Rodríguez AF, Navas P, de Miguel M (2010) Mitochondrial dysfunction in skin biopsies and blood mononuclear cells from two cases of fibromyalgia patients. Clin Biochem 43:1174–1176. https://doi.org/10.1016/j.clinbiochem.2010.06.013
Maldonado EN, Lemasters JJ (2014) ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion:78–84. https://doi.org/10.1038/mp.2011.182.doi
Brand MD, Nicholls DG (2015) Assessing mitochondrial dysfunction in cells. Biochem J 437:575. https://doi.org/10.1042/bj4370575u
Siegel MP, Kruse SE, Knowels G, Salmon A, Beyer R, Xie H, van Remmen H, Smith SR et al (2011) Reduced coupling of oxidative phosphorylation in vivo precedes electron transport chain defects due to mild oxidative stress in mice. PLoS One 6. https://doi.org/10.1371/journal.pone.0026963
Kwong JQ, Henning MS, Starkov AA, Manfredi G (2007) The mitochondrial respiratory chain is a modulator of apoptosis. J Cell Biol 179:1163–1177. https://doi.org/10.1083/jcb.200704059
Alrowayeh HN, Sabbahi MA, Etnyre B (2011) Similarities and differences of the soleus and gastrocnemius H-reflexes during varied body postures, foot positions, and muscle function: multifactor designs for repeated measures. BMC Neurology 11:. https://doi.org/10.1186/1471-2377-11-65
Bouitbir J, Haegler P, Singh F, Joerin L, Felser A, Duthaler U, Krähenbühl S (2016) Impaired Exercise Performance and Skeletal Muscle Mitochondrial Function in Rats with Secondary Carnitine Deficiency. Front Physiol 7. https://doi.org/10.3389/fphys.2016.00345
Cronin NJ, Avela J, Finni T, Peltonen J (2013) Differences in contractile behaviour between the soleus and medial gastrocnemius muscles during human walking. J Exp Biol 216:909–914. https://doi.org/10.1242/jeb.078196
Jacobs RA, Díaz V, Meinild AK, Gassmann M, Lundby C (2013) The C57Bl/6 mouse serves as a suitable model of human skeletal muscle mitochondrial function. Exp Physiol 98:908–921. https://doi.org/10.1113/expphysiol.2012.070037
Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K (2015) Oxidative stress in aging human skin. Biomolecules 5:545–589. https://doi.org/10.3390/biom5020545
Yao X, Li L, Kandhare A et al (2019) Attenuation of reserpine-induced fibromyalgia via ROS and serotonergic pathway modulation by fisetin, a plant flavonoid polyphenol. Exp Ther Med:1343–1355. https://doi.org/10.3892/etm.2019.8328
Availability of Data and Material
Not applicable.
Funding
This study was supported by the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul-FAPERGS and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Grant #16/2551-0000281-9); by FAPERGS (Grant #17/2551-0001082-5) (Brazil); by the CNPq (process #406098/2018-2); by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES/PROEX (process 23038.004173/2019-93, grant #0493/2019); and by PRONEM/FAPERGS/CNPQ (#16/2551-0000248-7). S.M.O is recipient of fellowship from CNPq (process #307220/2017-6) and E.S.B, M.F.P.F., D.F.G., and D.D.H. are recipients of fellowship from CAPES/PROEX (process #88887.185973/2018-00, #88882.182170/2018-01, #88882.182139/2018-01, and #88882.182131/2018-01, respectively). S.P.M.F. is recipient of fellowship from CNPq (process #134613/2017-1). We thank CNPq and CAPES for their fellowship support.
Author information
Authors and Affiliations
Contributions
Study concept and design: ESB and SMO. Acquisition of mitochondrial function data: ESB, DDH, and DFG. Acquisition of behaviours data: ESB, MFPF, and SPMF. Acquisition of biogenic amines data: ESB and RS. Analysis and interpretation of data: ESB, DDH, DFG, and SMO. Drafting and revising the content of the manuscript: ESB, RMA, CLDC, FAAS, and SMO. Study supervision: SMO.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Ethics Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. Experiments in animals were performed with the approval of the Institutional Committee for Animal Care and Use of the Federal University of Santa Maria (process numbers 3525100119/2019 and 5070100119/2019).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Headings
• Oligomycin (ATP insult) enhanced reserpine-induced pain and depressive-like behaviours.
• Coenzyme Q10 prevented reserpine-induced pain and depressive-like behaviours.
• Reserpine reduced electron transport and mitochondrial respiration in the muscles and spinal cord.
Rights and permissions
About this article
Cite this article
Brum, E.d., Fialho, M.F.P., Fischer, S.P.M. et al. Relevance of Mitochondrial Dysfunction in the Reserpine-Induced Experimental Fibromyalgia Model. Mol Neurobiol 57, 4202–4217 (2020). https://doi.org/10.1007/s12035-020-01996-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01996-1