Abstract
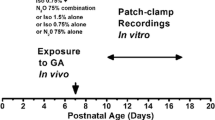
A large number of preclinical studies have established that general anesthetics (GAs) may cause neurodevelopmental toxicity in rodents and nonhuman primates, which is followed by long-term cognitive deficits. The subiculum, the main output structure of hippocampal formation, is one of the brain regions most sensitive to exposure to GAs at the peak of synaptogenesis (i.e., postnatal day (PND) 7). We have previously shown that subicular neurons exposed to GAs produce excessive amounts of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), which is a known modulator of neuronal excitability. To further explore the association between GA-mediated increase in ROS levels and long-term functional changes within subicular neurons, we sought to investigate the effects of ROS on excitability of these neurons using patch-clamp electrophysiology in acute rat brain slices. We hypothesized that both acute application of H2O2 and an early exposure (at PND 7) to GA consisting of midazolam (9 mg/kg), 70% nitrous oxide, and 0.75% isoflurane can affect excitability of subicular neurons and that superoxide dismutase and catalase mimetic, EUK-134, may reverse GA-mediated hyperexcitability in the subiculum. Our results using whole-cell recordings demonstrate that acute application of H2O2 has bidirectional effects on neuronal excitability: lower concentrations (0.001%, 0.3 mM) cause an excitatory effect, whereas higher concentrations (0.01%, 3 mM) inhibited neuronal firing. Furthermore, 0.3 mM H2O2 increased the average action potential frequency of subicular neurons by almost twofold, as assessed using cell-attach configuration. Finally, we found that preemptive in vivo administration of EUK-134 reduced GA-induced long-lasting hyperexcitability of subicular neurons ex vivo when studied in neonatal and juvenile rats. This finding suggests that the increase in ROS after GA exposure may play an important role in regulating neuronal excitability, thus making it an attractive therapeutic target for GA-induced neurotoxicity in neonates.




Similar content being viewed by others
References
Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. 23:876–882
Yon J-H, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V (2005) Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 135:815–827. https://doi.org/10.1016/j.neuroscience.2005.03.064
Loepke AW, Istaphanous GK, McAuliffe JJ et al (2009) The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 108:90–104. https://doi.org/10.1213/ane.0b013e31818cdb29
Squire LR, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253:1380–1386
Witter MP, Ostendorf RH, Groenewegen HJ (1990) Heterogeneity in the dorsal subiculum of the rat. Distinct neuronal zones project to different cortical and subcortical targets. Eur J Neurosci 2:718–725
Witter M (2006) Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res 174:251–264. https://doi.org/10.1016/j.bbr.2006.06.022
Atluri N, Joksimovic SM, Oklopcic A, Milanovic D, Klawitter J, Eggan P, Krishnan K, Covey DF et al (2018) A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. Br J Anaesth 120:768–778. https://doi.org/10.1016/j.bja.2017.12.039
DiGruccio MR, Joksimovic S, Joksovic PM et al (2015) Hyperexcitability of rat thalamocortical networks after exposure to general anesthesia during brain development. J Neurosci 35:1481–1492. https://doi.org/10.1523/JNEUROSCI.4883-13.2015
Lee CR, Patel JC, O’Neill B, Rice ME (2015) Inhibitory and excitatory neuromodulation by hydrogen peroxide: translating energetics to information. J Physiol 593:3431–3446. https://doi.org/10.1113/jphysiol.2014.273839
Orestes P, Bojadzic D, Lee J, Leach E, Salajegheh R, DiGruccio MR, Nelson MT, Todorovic SM (2011) Free radical signalling underlies inhibition of CaV3.2 T-type calcium channels by nitrous oxide in the pain pathway. J Physiol 589:135–148. https://doi.org/10.1113/jphysiol.2010.196220
Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V (2010) General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res 17:179–188. https://doi.org/10.1007/s12640-009-9088-z
Sanchez V, Feinstein SD, Lunardi N, Joksovic PM, Boscolo A, Todorovic SM, Jevtovic-Todorovic V (2011) General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology 115:992–1002. https://doi.org/10.1097/ALN.0b013e3182303a63
Boscolo A, Starr J, Sanchez et al (2012) The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis 45:1031. https://doi.org/10.1016/J.NBD.2011.12.022
Nunemaker CS, DeFazio RA, Moenter SM (2003) A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online 5:53–62. https://doi.org/10.1251/bpo46
Ohashi M, Hirano T, Watanabe K, Shoji H, Ohashi N, Baba H, Endo N, Kohno T (2016) Hydrogen peroxide modulates neuronal excitability and membrane properties in ventral horn neurons of the rat spinal cord. Neuroscience 331:206–220. https://doi.org/10.1016/j.neuroscience.2016.06.033
Garcia AJ, Khan SA, Kumar GK et al (2011) Hydrogen peroxide differentially affects activity in the pre-Bötzinger complex and hippocampus. J Neurophysiol 106:3045–3055. https://doi.org/10.1152/jn.00550.2010
Hyslop PA, Zhang Z, Pearson D V, Phebus LA (1995) Measurement of striatal H202 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H202 in vitro
FDA Drug Safety Communication. FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-results-new-warnings-about-using-general-anesthetics-and. Accessed 15 May 2019
Ostrowski TD, Hasser EM, Heesch CM, Kline DD (2014) H2O2 induces delayed hyperexcitability in nucleus tractus solitarii neurons. Neuroscience 262:53–69. https://doi.org/10.1016/j.neuroscience.2013.12.055
Gabrielli JDE, Brewer JB, Desmond JE, Glover GH (1997) Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science (80- ) 276:264–266. https://doi.org/10.1126/science.276.5310.264
Morris RGM, Schenk F, Tweedie F, Jarrard LE (1990) Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci 2:1016–1028
Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ (2000) Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry 57:349–356
Stafstrom CE (2005) The role of the subiculum in epilepsy and epileptogenesis. Epilepsy Curr 5:121–129. https://doi.org/10.1111/j.1535-7511.2005.00049.x
Palop JJ, Mucke L (2009) Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol 66:435. https://doi.org/10.1001/archneurol.2009.15
Noebels J (2011) A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia 52:39–46. https://doi.org/10.1111/j.1528-1167.2010.02909.x
Funding
This study was funded in part by grants from the National Institutes of Health (Grant No. R01GM102525 to S.M.T. and Grant No. R01GM118197 to V.J-T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were approved by the Institutional Animal Use and Care Committee of the University of Colorado Anschutz Medical Campus, Aurora, CO, and by the Animal Use and Care Committee of the University of Virginia, Charlottesville, VA. All experiments were done in accordance with the Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joksimovic, S.M., DiGruccio, M.R., Boscolo, A. et al. The Role of Free Oxygen Radicals in Lasting Hyperexcitability of Rat Subicular Neurons After Exposure to General Anesthesia During Brain Development. Mol Neurobiol 57, 208–216 (2020). https://doi.org/10.1007/s12035-019-01770-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01770-y