Abstract
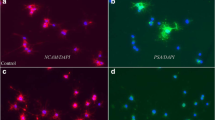
Early life exposure to general anesthetics can have neurotoxic consequences. Evidence indicates that xenon, a rare noble gas with anesthetic properties, may lessen neuronal damage under certain conditions. However, its potential neuroprotective properties, when used alone or in combination with other anesthetics, remain largely unknown. While it is difficult to verify the adverse effects of long duration anesthetic exposure in infants and children, the utilization of relevant non-clinical models (i.e., human-derived neural stem cells) may serve as a “bridging” model for evaluating the vulnerability of the nervous system. Neural stem cells, purchased from PhoenixSongs Biologicals, Inc., were guided to differentiate into neurons, astrocytes, and oligodendrocytes, which were then exposed to propofol (50 μM) for 16 h in the presence or absence of xenon (33%). Differentiation into cells of the neural lineage was confirmed by labelling with cell-specific markers, β-tubulin for neurons, glial fibrillary acidic protein (GFAP) for astrocytes, and galactocerebroside (GALC) for oligodendrocytes after 5 days of differentiation. The presence and severity of neural damage induced by anesthetic exposures were assessed by several methods, including the TUNEL assay, and immuno-histochemical measurements. Our data demonstrate that prolonged exposure to propofol results in a significant increase in the number of TUNEL-positive cells, indicating increased neural apoptosis. No significant changes were detected in the number of GFAP-positive astrocytes or GALC-positive oligodendrocytes. However, the number of β-tubulin-positive neurons was substantially reduced in the propofol-exposed cultures. Co-administration of xenon effectively blocked the propofol-induced neuronal damage/loss. No significant effects were observed when xenon was administered alone. The data indicate that prolonged exposure to propofol during development produces elevated levels of neuronal apoptosis in a human neural stem cell-derived model. However, sub-clinical, non-anesthetic concentrations of xenon, when used in combination with propofol, can prevent or ameliorate the toxic effects associated with prolonged anesthetic exposure. This is important as a more complete understanding of the neurotoxic mechanisms associated with a variety of clinically relevant anesthetic combinations becomes available. Protective approaches are critical for developing sound guidance on best practices for the use of these agents in the pediatric setting.








Similar content being viewed by others
References
Scallet AC, Schmued LC, Slikker W Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS et al (2004) Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci 81(2):364–370. https://doi.org/10.1093/toxsci/kfh224
Slikker W Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC et al (2007) Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci 98(1):145–158. https://doi.org/10.1093/toxsci/kfm084
Shi Q, Guo L, Patterson TA, Dial S, Li Q, Sadovova N, Zhang X, Hanig JP et al (2010) Gene expression profiling in the developing rat brain exposed to ketamine. Neuroscience 166(3):852–863. https://doi.org/10.1016/j.neuroscience.2010.01.007
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA et al (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33(2):220–230. https://doi.org/10.1016/j.ntt.2011.01.001
Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP et al (2007) Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology 106(4):746–753. https://doi.org/10.1097/01.anes.0000264762.48920.80
Cattano D, Valleggi S, Ma D, Kastsiuchenka O, Abramo A, Sun P, Cavazzana AO, Natale G et al (2008) Xenon induces transcription of ADNP in neonatal rat brain. Neurosci Lett 440(3):217–221. https://doi.org/10.1016/j.neulet.2008.05.086
Cattano D, Valleggi S, Cavazzana AO, Patel CB, Ma D, Maze M, Giunta F (2011) Xenon exposure in the neonatal rat brain: effects on genes that regulate apoptosis. Minerva Anestesiol 77(6):571–578
Sabir H, Bishop S, Cohen N, Maes E, Liu X, Dingley J, Thoresen M (2013) Neither xenon nor fentanyl induces neuroapoptosis in the newborn pig brain. Anesthesiology 119(2):345–357. https://doi.org/10.1097/ALN.0b013e318294934d
Liu F, Rainosek SW, Sadovova N, Fogle CM, Patterson TA, Hanig JP, Paule MG, Slikker W Jr et al (2014) Protective effect of acetyl-l-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology 42C:49–57. https://doi.org/10.1016/j.neuro.2014.03.011
Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ (2013) Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg 116(4):869–880. https://doi.org/10.1213/ANE.0b013e3182860fc9
Liu F, Patterson TA, Sadovova N, Zhang X, Liu S, Zou X, Hanig JP, Paule MG et al (2013) Ketamine-induced neuronal damage and altered N-methyl-D-aspartate receptor function in rat primary forebrain culture. Toxicol Sci 131(2):548–557. https://doi.org/10.1093/toxsci/kfs296
Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG et al (2006) Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci 91(1):192–201. https://doi.org/10.1093/toxsci/kfj144
Kahraman S, Zup SL, McCarthy MM, Fiskum G (2008) GABAergic mechanism of propofol toxicity in immature neurons. J Neurosurg Anesthesiol 20(4):233–240. https://doi.org/10.1097/ANA.0b013e31817ec34d
Johnson KM, Phillips M, Wang C, Kevetter GA (1998) Chronic phencyclidine induces behavioral sensitization and apoptotic cell death in the olfactory and piriform cortex. J Neurosci Res 52(6):709–722. https://doi.org/10.1002/(SICI)1097-4547(19980615)52:6<709::AID-JNR10>3.0.CO;2-U
Wang C, McInnis J, West JB, Bao J, Anastasio N, Guidry JA, Ye Y, Salvemini D et al (2003) Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J Pharmacol Exp Ther 304(1):266–271. https://doi.org/10.1124/jpet.102.041798
Cattano D, Valleggi S, Cavazzana AO, Patel CB, Ma D, Giunta F (2011) Xenon exposure in the neonatal rat brain: effects on genes that regulate apoptosis. Minerva Anestesiol 77(6):571–578
Yan Y, Qiao S, Kikuchi C, Zaja I, Logan S, Jiang C, Arzua T, Bai X (2017) Propofol induces apoptosis of neurons but not astrocytes, oligodendrocytes, or neural stem cells in the neonatal mouse hippocampus. Brain Sci 7(10). https://doi.org/10.3390/brainsci7100130
Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A (2013) Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 110(Suppl 1):i29–i38. https://doi.org/10.1093/bja/aet173
Wang C, Liu F, Patterson TA, Paule MG, Slikker W Jr (2013) Preclinical assessment of ketamine. CNS Neurosci Ther 19:448–453. https://doi.org/10.1111/cns.12079
Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, Hanig JP, Paule MG et al (2011) Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol 33(5):592–597. https://doi.org/10.1016/j.ntt.2011.06.003
Zou X, Sadovova N, Patterson TA, Divine RL, Hotchkiss CE, Ali SF, Hanig JP, Paule MG et al (2008) The effects of L-carnitine on the combination of, inhalation anesthetic-induced developmental, neuronal apoptosis in the rat frontal cortex. Neuroscience 151(4):1053–1065. https://doi.org/10.1016/j.neuroscience.2007.12.013
Campagna JA, Miller KW, Forman SA (2003) Mechanisms of actions of inhaled anesthetics. N Engl J Med 348(21):2110–2124. https://doi.org/10.1056/NEJMra021261
Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP (2007) Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology 107(5):756–767. https://doi.org/10.1097/01.anes.0000287061.77674.71
Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J (2008) Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 39(4):1307–1313. https://doi.org/10.1161/STROKEAHA.107.499822
Ma D, Wilhelm S, Maze M, Franks NP (2002) Neuroprotective and neurotoxic properties of the ‘inert’ gas, xenon. Br J Anaesth 89(5):739–746
Chakkarapani E, Thoresen M, Hobbs CE, Aquilina K, Liu X, Dingley J (2009) A closed-circuit neonatal xenon delivery system: a technical and practical neuroprotection feasibility study in newborn pigs. Anesth Analg 109(2):451–460. https://doi.org/10.1213/ane.0b013e3181aa9550
Dingley J, Tooley J, Porter H, Thoresen M (2006) Xenon provides short-term neuroprotection in neonatal rats when administered after hypoxia-ischemia. Stroke 37(2):501–506. https://doi.org/10.1161/01.STR.0000198867.31134.ac
Natale G, Cattano D, Abramo A, Forfori F, Fulceri F, Fornai F, Paparelli A, Giunta F (2006) Morphological evidence that xenon neuroprotects against N-methyl-DL-aspartic acid-induced damage in the rat arcuate nucleus: a time-dependent study. Ann N Y Acad Sci 1074:650–658. https://doi.org/10.1196/annals.1369.063
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The information in these materials is not a formal dissemination of information by the FDA and does not represent agency position or policy.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, F., Liu, S., Patterson, T.A. et al. Protective Effects of Xenon on Propofol-Induced Neurotoxicity in Human Neural Stem Cell-Derived Models. Mol Neurobiol 57, 200–207 (2020). https://doi.org/10.1007/s12035-019-01769-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01769-5