Abstract
Loss of nigrostriatal projections by axonal degeneration is a key early event in Parkinson’s disease (PD) pathophysiology, being accountable for the lack of dopamine in the nigrostriatal system and resulting in motor symptoms such as bradykinesia, rigidity, and tremor. Since autophagy is an important mechanism contributing to axonal degeneration, we aimed to evaluate the effects of competitive autophagy inhibition in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD in vivo. Adeno-associated viral vector (AAV)–mediated overexpression of a dominant-negative form of the unc-51 like autophagy-initiating kinase (ULK1.DN) in the substantia nigra was induced 3 weeks before MPTP treatment. Analysis of motor behavior demonstrated a significant improvement of ULK1.DN expressing mice after MPTP treatment. Immunohistochemical analyses of dopaminergic nigral neurons and nigrostriatal projections revealed a significant protection from MPTP-induced neurotoxicity after ULK1.DN expression. Western blot analysis linked these findings to an activation of mTOR signaling. Taken together, our results indicate that expression of ULK1.DN can attenuate MPTP-induced axonal neurodegeneration, suggesting that ULK1 could be a promising novel target in the treatment of PD.






Similar content being viewed by others
Abbreviations
- AAD:
-
Acute axonal degeneration
- AAV:
-
Adeno-associated virus
- AMPK:
-
AMP-activated protein kinase
- AP:
-
Anterior posterior
- ATG:
-
Autophagy related
- DA:
-
Dopamine
- DOPAC:
-
Dihydroxyphenylacetic acid
- DV:
-
Dorsoventral
- FIP200:
-
FAK family interacting protein of 200 kDa
- HPLC:
-
High-performance liquid chromatography
- HVA:
-
Homovanillic acid
- LC3:
-
Microtubule-associated proteins 1A/1B light chain 3B
- MPP +:
-
1-Methyl-4-phenylpyridinium.
- ML:
-
Mediolateral
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mTOR:
-
Mechanistic target of rapamycin kinase
- p62:
-
Sequestosome 1/p62
- PD:
-
Parkinson’s disease
- SN:
-
Substantia nigra
- SNpc:
-
Substantia nigra pars compacta
- TH:
-
Tyrosine hydroxylase
- ULK1:
-
Unc-51 like autophagy activating kinase
- ULK1.DN:
-
Dominant-negative ULK1.
References
Salat D, Noyce AJ, Schrag A, Tolosa E (2016) Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol 15:637–648. https://doi.org/10.1016/S1474-4422(16)00060-0
Burke RE, O’Malley K (2013) Axon degeneration in Parkinson’s disease. Exp Neurol 246:72–83. https://doi.org/10.1016/j.expneurol.2012.01.011
Lingor P, Koch JC, Tönges L, Bähr M (2012) Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res 349:289–311. https://doi.org/10.1007/s00441-012-1362-3
Tagliaferro P, Burke RE (2016) Retrograde axonal degeneration in Parkinson disease. J Park Dis 6:1–15. https://doi.org/10.3233/JPD-150769
Banerjee R, Beal MF, Thomas B (2010) Autophagy in neurodegenerative disorders: Pathogenic roles and therapeutic implications. Trends Neurosci 33:541–549. https://doi.org/10.1016/j.tins.2010.09.001
Nah J, Yuan J, Jung Y-K (2015) Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cell 38:381–389. https://doi.org/10.14348/molcells.2015.0034
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13:805–811. https://doi.org/10.1038/nn.2575
Koch JC, Knöferle J, Tönges L, Ostendorf T, Bähr M, Lingor P (2010) Acute axonal degeneration in vivo is attenuated by inhibition of autophagy in a calcium-dependent manner. Autophagy 6:658–659. https://doi.org/10.4161/auto.6.5.12188
Knöferle J, Koch JC, Ostendorf T et al (2010) Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A 107:6064–6069. https://doi.org/10.1073/pnas.0909794107
Ribas VT, Schnepf B, Challagundla M, Koch JC, Bähr M, Lingor P (2015) Early and sustained activation of autophagy in degenerating axons after spinal cord injury. Brain Pathol 25:157–170. https://doi.org/10.1111/bpa.12170
Cheng H-C, Kim SR, Oo TF, Kareva T, Yarygina O, Rzhetskaya M, Wang C, During M et al (2011) Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci 31:2125–2135. https://doi.org/10.1523/JNEUROSCI.5519-10.2011
Dagda RK, Das BT, Janda E (2013) How parkinsonian toxins dysregulate the autophagy machinery. Int J Mol Sci 14:22163–22189. https://doi.org/10.3390/ijms141122163
Janda E, Isidoro C, Carresi C, Mollace V (2012) Defective autophagy in Parkinson’s disease: Role of oxidative stress. Mol Neurobiol 46:639–661. https://doi.org/10.1007/s12035-012-8318-1
Murphy KE, Gysbers AM, Abbott SK, Spiro AS, Furuta A, Cooper A, Garner B, Kabuta T et al (2015) Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov Disord 30:1639–1647. https://doi.org/10.1002/mds.26141
Zhang L, Dong Y, Xu X, Xu Z (2012) The role of autophagy in Parkinson’s disease. Neural Regen Res 7:141–145. https://doi.org/10.3969/j.issn.1673-5374.2012.02.011
Kaur J, Debnath J (2015) Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 16:461–472. https://doi.org/10.1038/nrm4024
Chan EYW, Longatti A, McKnight NC, Tooze SA (2009) Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol 29:157–171. https://doi.org/10.1128/MCB.01082-08
Vahsen BF, Ribas VT, Lenz C, Roser AE, Michel U, Urlaub H et al (2018) Role of autophagic protein ULK1 in axonal degeneration and regeneration in cortical neurons in vitro. In: 11th FENS Forum of Neuroscience, 7–11 July 2018, Berlin, Germany p Poster C118. https://ep70.eventpilot.us/web/page.php?page=IntHtml&project=FENS18&id=abstract_34677
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ et al (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 6:973–985. https://doi.org/10.1038/sj.gt.3300938
Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I et al (2008) Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci 105:7827–7832. https://doi.org/10.1073/pnas.0802866105
Tatenhorst L, Tönges L, Saal K-A, Koch JC, Szegő ÉM, Bähr M, Lingor P (2014) Rho kinase inhibition by fasudil in the striatal 6-hydroxydopamine lesion mouse model of Parkinson disease. J Neuropathol Exp Neurol 73:770–779. https://doi.org/10.1097/NEN.0000000000000095
Saal K-A, Koch JC, Tatenhorst L, Szegő ÉM, Ribas VT, Michel U, Bähr M, Tönges L et al (2015) AAV.shRNA-mediated downregulation of ROCK2 attenuates degeneration of dopaminergic neurons in toxin-induced models of Parkinson’s disease in vitro and in vivo. Neurobiol Dis 73:150–162. https://doi.org/10.1016/j.nbd.2014.09.013
Tönges L, Frank T, Tatenhorst L et al (2012) Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson’s disease. Brain 135:3355–3370. https://doi.org/10.1093/brain/aws254
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39:777–787
Tatenhorst L, Eckermann K, Dambeck V, Fonseca-Ornelas L, Walle H, Lopes da Fonseca T, Koch JC, Becker S et al (2016) Fasudil attenuates aggregation of α-synuclein in models of Parkinson’s disease. Acta Neuropathol Commun 4:39. https://doi.org/10.1186/s40478-016-0310-y
Bhimani AD, Kheirkhah P, Arnone GD, Nahhas CR, Kumar P, Wonais M, Hidrogo H, Aguilar E et al (2017) Functional gait analysis in a spinal contusion rat model. Neurosci Biobehav Rev 83:540–546. https://doi.org/10.1016/j.neubiorev.2017.09.007
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy ( 3rd edition ). Autophagy 12:1–222. https://doi.org/10.1080/15548627.2015.1100356
Kim J, Kundu M, Viollet B, Guan K-L (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141. https://doi.org/10.1038/ncb2152
Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16:345–357. https://doi.org/10.1038/nrn3961
Zhu J-H, Guo F, Shelburne J et al (2003) Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol 13:473–481
Beilina A, Cookson MR (2016) Genes associated with Parkinson’s disease: Regulation of autophagy and beyond. J Neurochem 139:91–107. https://doi.org/10.1111/jnc.13266
Kurowska Z, Kordower JH, Stoessl AJ, Burke RE, Brundin P, Yue Z, Brady ST, Milbrandt J et al (2016) Is axonal degeneration a key early event in Parkinson’s disease? J Park Dis 6:703–707. https://doi.org/10.3233/JPD-160881
Ribas VT, Koch JC, Michel U, Bähr M, Lingor P (2016) Attenuation of axonal degeneration by Calcium Channel inhibitors improves retinal ganglion cell survival and regeneration after optic nerve crush. Mol Neurobiol 54:72–86. https://doi.org/10.1007/s12035-015-9676-2
Zhang J-N, Michel U, Lenz C, Friedel CC, Köster S, d’Hedouville Z, Tönges L, Urlaub H et al (2016) Calpain-mediated cleavage of collapsin response mediator protein-2 drives acute axonal degeneration. Sci Rep 6:37050. https://doi.org/10.1038/srep37050
Miki Y, Tanji K, Mori F, Utsumi J, Sasaki H, Kakita A, Takahashi H, Wakabayashi K (2016) Alteration of upstream autophagy-related proteins (ULK1, ULK2, Beclin1, VPS34 and AMBRA1) in Lewy body disease. Brain Pathol 26:359–370. https://doi.org/10.1111/bpa.12297
Miki Y, Shimoyama S, Kon T, Ueno T, Hayakari R, Tanji K, Matsumiya T, Tsushima E et al (2018) Alteration of autophagy-related proteins in peripheral blood mononuclear cells of patients with Parkinson’s disease. Neurobiol Aging 63:33–43. https://doi.org/10.1016/j.neurobiolaging.2017.11.006
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M (2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76:1265–1274
Huang D, Xu J, Wang J, Tong J, Bai X, Li H, Wang Z, Huang Y et al (2017) Dynamic changes in the nigrostriatal pathway in the MPTP mouse model of Parkinson’s disease. Parkinsons Dis 2017:1–7. https://doi.org/10.1155/2017/9349487
Rommelfanger KS (2011) Extrastriatal dopaminergic circuits of the basal ganglia. Front Neuroanat 4:1–17. https://doi.org/10.3389/fnana.2010.00139
Pilotto A, di Cola FS, Premi E et al (2019) Extrastriatal dopaminergic and serotonergic pathways in Parkinson’s disease and in dementia with Lewy bodies: a 123I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging 46:1642–1651. https://doi.org/10.1007/s00259-019-04324-5
Chan EYW, Kir S, Tooze SA (2007) siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem 282:25464–25474. https://doi.org/10.1074/jbc.M703663200
Alers S, Loffler a. S, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32:2–11. https://doi.org/10.1128/MCB.06159-11
Toda H, Mochizuki H, Flores R, Josowitz R, Krasieva TB, LaMorte VJ, Suzuki E, Gindhart JG et al (2008) UNC-51/ATG1 kinase regulates axonal transport by mediating motor-cargo assembly. Genes Dev 22:3292–3307. https://doi.org/10.1101/gad.1734608
Chu CT, Zhu J, Dagda R (2007) Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy 3:663–666
Zhang H, Duan C, Yang H (2015) Defective autophagy in Parkinson’s disease: Lessons from genetics. Mol Neurobiol 51:89–104. https://doi.org/10.1007/s12035-014-8787-5
Li Y, Zhang J, Yang C (2015) UNC-51-like kinase 1 blocks S6k1 phosphorylation contributes to neurodegeneration in Parkinson’s disease model in vitro. Biochem Biophys Res Commun 459:196–200. https://doi.org/10.1016/j.bbrc.2015.02.008
Lee S-B, Kim H-T, Yang HO, Jang W (2018) Anodal transcranial direct current stimulation prevents methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity by modulating autophagy in an in vivo mouse model of Parkinson’s disease. Sci Rep 8:15165. https://doi.org/10.1038/s41598-018-33515-7
Wen Z, Zhang J, Tang P, Tu N, Wang K, Wu G (2018) Overexpression of miR-185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol Med Rep 17:131–137. https://doi.org/10.3892/mmr.2017.7897
Jang Y chul, Hwang DJ, Koo JH, et al (2018) Association of exercise-induced autophagy upregulation and apoptosis suppression with neuroprotection against pharmacologically induced Parkinson’s disease. J Exerc Nutr Biochem 22:1–8. https://doi.org/10.20463/jenb.2018.0001
Ouyang L, Zhang L, Zhang S, Yao D, Zhao Y, Wang G, Fu L, Lei P et al (2018) Small-molecule activator of UNC-51-like kinase 1 (ULK1) that induces cytoprotective autophagy for Parkinson’s disease treatment. J Med Chem 61:2776–2792. https://doi.org/10.1021/acs.jmedchem.7b01575
Pupyshev AB, Tikhonova MA, Akopyan AA, Tenditnik MV, Dubrovina NI, Korolenko TA (2019) Therapeutic activation of autophagy by combined treatment with rapamycin and trehalose in a mouse MPTP-induced model of Parkinson’s disease. Pharmacol Biochem Behav 177:1–11. https://doi.org/10.1016/j.pbb.2018.12.005
Tang F, Hu P, Yang Z, Xue C, Gong J, Sun S, Shi L, Zhang S et al (2017) SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways. Oncol Rep 37:3449–3458. https://doi.org/10.3892/or.2017.5635
Egan DF, Chun MGH, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D et al (2015) Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell 59:285–297. https://doi.org/10.1016/j.molcel.2015.05.031
Acknowledgments
The authors thank Elisabeth Barski, Sabine Ceramella, and Barbara Müller for excellent technical support. We thank Sharon A. Tooze (Francis Crick Institute, London, UK) for providing the ULK1.DN plasmid.
Funding
D.B. and B.F.V. were supported by a scholarship from the Department of Neurology, University Medical Center Göttingen. V.T.R. was a fellow of the National Council for Scientific and Technological Development (CNPq), Brazil. L.T., V.D., M.B. and P.L. received funding from the Cluster of Excellence and DFG Research Center Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB), Göttingen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in this study involving animals were in accordance with the ethical standards and followed the regulations of the animal research council at the University Medical Center Göttingen and legislation of the State of Lower Saxony, Germany (33.9-42502-04-16/2239).
This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Fig. S1
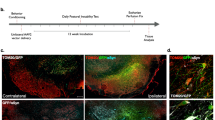
Injection of AAV.ULK1.DN into the substantia nigra pars compacta (SNpc). Adeno-associated viral vectors (AAV) were injected into the SNpc relative to Bregma at anterior posterior: −3.0 mm, mediolateral: −1.2 mm and dorsoventral: −4.5 mm (a). Representative overview of mCherry expression after AAV injection into the SNpc of the mouse brain. Cell nuclei were counterstained with DAPI to visualize brain structures. Scale bar: 1 mm (b). Representative photomicrograph of the mouse midbrain region visualizing the co-localization of mCherry expression and tyrosine hydroxylase (TH) expression in the SNpc after AAV injection. Cell nuclei were counterstained with DAPI. Scale bar: 500 μm (c). (PNG 1426 kb)
Fig. S2
Immunohistochemical analysis of p62 in the substantia nigra pars compacta (SNpc). Representative photomicrographs of the SNpc in the treatment groups CTRL PBS, ULK1.DN PBS, CTRL MPTP and ULK1.DN MPTP show an increase of p62 protein after ULK1.DN expression. White arrowheads indicate p62 signal. Scale bar: 50 μm. (PNG 1120 kb)
Fig. S3
Immunohistochemical analysis of mTOR in the substantia nigra pars compacta (SNpc). Representative photomicrographs of the SNpc in the treatment groups CTRL PBS, ULK1.DN PBS, CTRL MPTP and ULK1.DN MPTP show an increase of mTOR protein after ULK1.DN expression. Scale bar: 50 μm. (PNG 1471 kb)
ESM 4
(DOCX 16 kb)
ESM 5
(DOCX 14 kb)
ESM 6
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Balke, D., Tatenhorst, L., Dambeck, V. et al. AAV-Mediated Expression of Dominant-Negative ULK1 Increases Neuronal Survival and Enhances Motor Performance in the MPTP Mouse Model of Parkinson’s Disease. Mol Neurobiol 57, 685–697 (2020). https://doi.org/10.1007/s12035-019-01744-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01744-0