Abstract
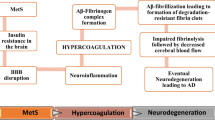
It has been well established in recent research that there is a strong correlation between metabolic syndrome (MetS) and Alzheimer’s disease (AD). However, the knowledge of exact mechanistic behind this association remains elusive. It has been reported in recent studies that inflammation and hypercoagulation are pivotal to the pathophysiology of MetS-induced AD. It is rather captivating that aberrant Wnt signaling pathway has been found to be implicated in each of the four conditions, i.e., inflammation, hypercoagulation, MetS, and AD. Deregulation of Wnt signaling has been affiliated with numerous brain pathologies, including Alzheimer’s disease and insulin resistance. In recent past, it has been proposed that the Wnt pathway can act as a central integrator of metabolic signals from peripheral organs to the brain, which would constitute a unique character for Wnt signaling in glucose metabolism. The review educates in what way distinct components of Wnt signaling impact effector mediators of inflammation, hypercoagulation, which in turn decelerate the progression of AD in MetS. Furthermore, components of Wnt signaling, namely, Wnt3a and GSK-3β, interlink MetS and AD. The review opines a contemporary hypothesis that Wnt signaling is implicated in the pathogenesis of MetS-induced AD via impacting inflammation and coagulation. Hence, targeting Wnt signaling could be a novel approach to halt the progression of MetS-linked AD.


Similar content being viewed by others
References
Husain I, Akhtar M, Madaan T, Vohora D, Abdin MZ, Islamuddin M, Najmi AK (2018) Tannins enriched fraction of Emblica officinalis fruits alleviates high-salt and cholesterol diet-induced cognitive impairment in rats via Nrf2–ARE pathway. Front Pharmacol 9:23
Motamedi S, Karimi I, Jafari F (2017) The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): kill two birds with one stone. Metab Brain Dis 32:651–665. https://doi.org/10.1007/s11011-017-9997-0
Pugazhenthi S (2017) Metabolic syndrome and the cellular phase of Alzheimer’s disease. Prog Mol Biol Transl Sci 146:243–258. https://doi.org/10.1016/bs.pmbts.2016.12.016
Suidan GL, Singh PK, Patel-Hett S, Chen ZL, Volfson D, Yamamoto-Imoto H, Norris EH, Bell RD et al (2018) Abnormal clotting of the intrinsic/contact pathway in Alzheimer disease patients is related to cognitive ability. Blood Adv 2(9):954–963
Cortes-Canteli M, Mattei L, Richards AT, Norris EH, Strickland S (2015) Fibrin deposited in the Alzheimer’s disease brain promotes neuronal degeneration. Neurobiol Aging 36(2):608–617. https://doi.org/10.1016/j.neurobiolaging.2014.10.030
Ma Q, Jiang L, Mao J, Xu W, Huang M (2018) Vildagliptin prevents cognitive deficits and neuronal apoptosis in a rat model of Alzheimer’s disease. Mol Med Rep 17:4113–4119. https://doi.org/10.3892/mmr.2017.8289
Esmon CT (2005) The interactions between inflammation and coagulation. Br J Haematol 131:417–430
Abhishek RA, Ansari SA, Das K, Prasad R, Bhattacharya A (2017) Coagulation factor VIIa–mediated protease-activated receptor 2 activation leads to β-catenin accumulation via the AKT/GSK3β pathway and contributes to breast cancer progression. J Biol Chem 292(33):13688–13701. https://doi.org/10.1074/jbc.M116.764670
Abou Ziki MD, Mani A (2018) The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr Res. https://doi.org/10.1016/j.nutres.2018.06.009
Prestwich TC, MacDougald OA (2007) Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19:612–617
Ciani L, Salinas PC (2005) WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci 6(5):351–362
Tapia-Rojas C, Inestrosa NC (2018) Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen Res 13(10):1705–1710. https://doi.org/10.4103/1673-5374.238606
Hartz AMS, Bauer B, Soldner ELB, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U et al (2012) Amyloid-훽 contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 43(2):514–523
Ying Z, Nan-qu H, Fei Y, Hai J, Shaoyu Z, Jing-shan S, Feng J (2018) Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav Brain Res. https://doi.org/10.1016/j.bbr.2017.11.015
Zhang W, Xin L, Lu Y (2017) Integrative analysis to identify common genetic markers of metabolic syndrome, dementia, and diabetes. Med Sci Monit 23:5885–5891. Published 2017. https://doi.org/10.12659/MSM.905521
Jayaraman A, Pike CJ (2014) Alzheimer’s disease and type 2 diabetes: multiple mechanisms contribute to interactions. Curr Diab Rep 14(4):476
Dijk V, Gertjan et al (2015) Integrative neurobiology of metabolic diseases, neuroinflammation, and neurodegeneration. Front Neurosci 9:173. https://doi.org/10.3389/fnins.2015.00173
Contreras DL, Carvajal K, Rios DT, Bocanegra DF, Peña VC (2014) Oxidative stress and metabolic syndrome: cause or consequence of Alzheimer’s disease? Oxidative Med Cell Longev. 497802. https://doi.org/10.1155/2014/497802
Gutierrez ER, Arenas GM, Trevino S, Espinosa B, Chavez R, Rojas K et al (2017) Alzheimer’s disease and metabolic syndrome: a link from oxidative stress and inflammation to neurodegeneration. Synapse. 71:e21990. https://doi.org/10.1002/syn.21990
Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 137:47–59
Foster EM, Dangla-Valls A, Lovestone S, Ribe EM, Buckley NJ (2019) Clusterin in Alzheimer’s disease: mechanisms, genetics, and lessons from other pathologies. Front Neurosci 13:164
Won JC, Park CY, Oh SW, Lee ES, Youn BS, Kim MS (2014) Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS One 9(7):e103351
Seo JA, Kang MC, Ciaraldi TP, Kim SS, Park KS, Choe C, Hwang WM, Lim DM et al (2018) Circulating ApoJ is closely associated with insulin resistance in human subjects. Metabolism. 78:155–166. https://doi.org/10.1016/j.metabol.2017.09.014
Pinto D, Clevers H (2005) Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306:357–363
Sharma RP et al (1976) Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol 48:461–465
Niehrs C (2012) The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13:767–779
Jeong WJ, Ro EJ, Kang-Yell Choi KY (2018) Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. npj Precision Oncol 2:5. https://doi.org/10.1038/s41698-018-0049-y
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P (1996) Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 272:1023–1026
Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316:1619–1622
Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J et al (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135:367–375
Metcalfe C, Mendoza-Topaz C, Mieszczanek J, Bienz M (2010) Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX-dependent polymerization. J Cell Sci 123:1588–1599
Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH et al (2001) Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A 98:14973–14978
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B et al (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426
Willert J, Epping M, Pollack JR, Brown PO, Nusse R (2002) A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2:8
Hendrickx M, Leyns L (2008) Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Different 50:229–243
Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C (2001) LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411:321–325
Li L, Mao J, Sun L, Liu W, Wu D (2002) Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem 277:5977–5981
Mao B, Niehrs C (2003) Kremen2 modulates Dickkopf2 activity during Wnt/lRP6 signaling. Gene 302:179–183
Ma B, Hottiger MO (2016) Crosstalk between Wnt/β-catenin and NF-κB signaling pathway during inflammation. Front Immunol 7:378. https://doi.org/10.3389/fimmu.2016.00378
Ma B, van Blitterswijk CA, Karperien M (2012) A Wnt/beta-catenin negative feed-back loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum 64(8):2589–2600. https://doi.org/10.1002/art.34425
Sun J, Hobert ME, Duan Y, Rao AS, He TC, Chang EB, Madara JL (2005) Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 289(1):G129–G137. https://doi.org/10.1152/ajpgi.00515.2004
Duan Y, Liao AP, Kuppireddi S, Ye Z, Ciancio MJ, Sun J (2007) Beta-catenin activity negatively regulates bacteria-induced inflammation. Lab Investig 87(6):613–624. https://doi.org/10.1038/labinvest.3700545
Die L, Yan P, Jun Jiang Z, Min Hua T, Cai W, Xing L (2012) Glycogen synthase kinase-3 beta inhibitor suppresses Porphyromonas gingivalis lipopolysaccharide-induced CD40 expression by inhibiting nuclear factor-kappa B activation in mouse osteoblasts. Mol Immunol 52(1):38–49. https://doi.org/10.1016/j.molimm.2012.04.005
Kim SJ, Lim JY, Lee JN, Choe SK, Kim YI, Song SR, Cho M, So HS et al (2014) Activation of beta-catenin by inhibitors of glycogen synthase kinase-3 ameliorates cisplatin-induced cytotoxicity and pro-inflammatory cytokine expression in HEI-OC1 cells. Toxicology 320:74–82. https://doi.org/10.1016/j.tox.2014.01.013
Hao HP, Wen LB, Li JR, Wang Y, Ni B, Wang R, Wang X, Sun MX et al (2015) LiCl inhibits PRRSV infection by enhancing Wnt/beta-catenin pathway and suppressing inflammatory responses. Antivir Res 117:99–109. https://doi.org/10.1016/j.antiviral.2015.02.010
Ke B, Shen XD, Kamo N, Ji H, Yue S, Gao F, Busuttil RW, Kupiec-Weglinski JW (2013) Beta-catenin regulates innate and adaptive immunity in mouse liver ischemia-reperfusion injury. Hepatology 57(3):1203–1214. https://doi.org/10.1002/hep.26100
Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B (2010) Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329(5993):849–853. https://doi.org/10.1126/science.1188510
Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, Yamagoe S, Colnot S et al (2012) Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest 122(2):586–599. https://doi.org/10.1172/JCI43937
Martin M, Rehani K, Jope RS, Michalek SM (2005) Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 6(8):777–784. https://doi.org/10.1038/ni1221
Levi M, van der Poll T, Buller HR (2004) Bidirectional relation between inflammation and coagulation. Circulation 109:2698–2704
Levi M, Cate H (1999) Disseminated intravascular coagulation. N Engl J Med 341:586–592
Shu F, Kobayashi H, Fukudome K, Tsuneyoshi N, Kimoto M, Terao T (2000) Activated protein C suppresses tissue factor expression on U937 cells in the endothelial protein C receptor-dependent manner. FEBS Lett 477:208–212
Bajzar L, Nesheim ME, Tracy PB (1996) The profibrinolytic effect of activated protein C in clots formed from plasma is TAFI-dependent. Blood 88:2093–2100
Raaphorst J, Groeneveld AB, Bossink AW, Hack CE (2001) Early inhibition of activated fibrinolysis predicts microbial infection, shock and mortality in febrile medical patients. Thromb Haemost 86:543–549
White B, Schmidt M, Murphy C, Livingstone W, O’Toole D, Lawler M, O’Neill L, Kelleher D et al (2000) Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kB and tumour necrosis factor alpha1 production in the THP-1 monocytic cell line. Br J Haematol 110:130–134
Toltl LJ, Beaudin S, Liaw PC, the Canadian Critical Care Translational Biology Group (2008) Activated protein C up-regulates IL-10 and inhibits tissue factor in blood monocytes. J Immunol 181:2165–2173
Brueckmann M, Hoffmann U, de Rossi L, Weiler HM, Liebe V, Lang S, Kaden JJ, Borggrefe M et al (2004) Activated protein C inhibits the release of macrophage inflamatory protein-1-alpha from THP-1 cells and from human monocytes. Cytokine 26:106–113
Feistritzer C, Mosheimer BA, Sturn DH, Riewald M, Patsch JR, Wiedermann CJ (2006) Endothelial protein C receptor-dependent inhibition of migration of human lymphocytes by protein C involves epidermal growth factor receptor. J Immunol 176:1019–1025
Bernard GR, Vincent JL, Laterre PF, LaRosa S, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE et al (2001) Efficacy and safety of recombinant human activated protein c for severe sepsis. N Engl J Med 344:699–709
Taylor FB, Chang A, Esmon CT et al (1987) Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest 79:918–925
Brueckmann M, Hoffmann U, Dvortsak E et al (2004) Drotrecogin alfa (activated) inhibits NF-kappa B activation and MIP-1-alpha release from isolated mononuclear cells of patients with severe sepsis. Inflamm Res 53:528–533
Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O’Brien J, Gruber M, Zarini S et al (2004) Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood 104:3878–3885
Pereira C, Schaer DJ, Bachli EB (2008) Wnt5A/CaMKII signalling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol 28:504–510
Kopelman P (2000) Obesity as a medical problem. Nature 404:635–643
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald O (2000) Inhibition of adipogenesis by Wnt signaling. Science 289:950–953
Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A (2009) Adipogenesis and WNT signalling. Trends Endocrinol Metab 20:16–24
Moon RT, Bowerman B, Boutros M, Perrimon N (2002) The promise and perils of Wnt signaling through beta-catenin. Science 296:1644–1646
Choi OM, Cho Y-H, Choi S, Lee S-H, Seo SH, Kim H-Y et al (2014) The small molecule indirubin-30-oxime activates Wnt/b-catenin signaling and inhibits adipocyte differentiation and obesity. Int J Obes 38:1044–1052. https://doi.org/10.1038/ijo.2013.209
Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Miyatsuka T, Matsuhisa M et al (2005) Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med (Berlin, Germany) 83:429–439
Grimes CA, Jope RS (2001) CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem 78:1219–1232
Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JEB (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275:10761–10766
Tanioka T, Tamura Y, Fukaya M, Shinozaki S, Mao J, Kim M, Shimizu N, Kitamura T et al (2011) Inducible nitric-oxide synthase and nitric oxide donor decrease insulin receptor substrate-2 protein expression by promoting proteasome-dependent degradation in pancreatic betacells: involvement of glycogen synthase kinase-3beta. J Biol Chem 286:29388–29396
Gagliardino JJ, Del Zotto H, Massa L, Flores LE, Borelli MI (2003) Pancreatic duodenal homeobox-1 and islet neogenesis-associated protein: a possible combined marker of activateable pancreatic cell precursors. J Endocrinol 177:249–259
Brownlee M (2003) A radical explanation for glucose-induced beta cell dysfunction. J Clin Invest 112:1788–1790
Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Meneur C et al (2010) Conditional ablation of Gsk-3beta in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 53:2600–2610
Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL et al (2006) Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet 43:798–803
Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S et al (2007) LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315:1278–1282
Singh R, Smith E, Fathzadeh M, Liu W, Go GW, Subrahmanyan L, Faramarzi S, McKenna W et al (2013) Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Hum Mutat 34:1221–1225
Singh R, De Aguiar RB, Naik S et al (2013) LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab 17:197–209
Elghazi L, Gould AP, Weiss AJ, Barker DJ, Callaghan J, Opland D, Myers M, Cras-Méneur C et al (2012) Importance of b-catenin in glucose and energy homeostasis. Sci Rep 2:693
Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX et al (2003) Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A 100:229–234
Liu W, Singh R, Choi CS, Lee HY, Keramati AR, Samuel VT, Lifton RP, Shulman GI et al (2012) Low density lipoprotein (LDL) receptor-related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. J Biol Chem 287:7213–7223
Liu W, Mani S, Davis NR, Sarrafzadegan N, Kavathas PB, Mani A (2008) Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ Res 103:1280–1288
Ye ZJ, Go GW, Singh R, Liu W, Keramati AR, Mani A (2012) LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake. J Biol Chem 287:1335–1344
Go GW, Srivastava R, Hernandez-Ono A, Gang G, Smith SB, Booth CJ, Ginsberg HN, Mani A (2014) The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue. Cell Metab 19:209–220
Xu G, Emmons R, Hernández-Saavedra D, Kriska A, Pan YX, Chen H (2017) Regulation of gene expression of Wnt signaling pathway by dietary high fat and effects on colon epithelia of male mice. Nutrition 31(1)
Husain I, Khan S, Khan S, Madaan T, Kumar S, Najmi AK (2019 Apr) Unfolding the pleiotropic facades of rosuvastatin in therapeutic intervention of myriads of neurodegenerative disorders. Clin Exp Pharmacol Physiol 46(4):283–291
Bu’ee L, Troquier L, Burnouf S et al (2010) From tau phosphorylation to tau aggregation: what about neuronal death? Biochem Soc Trans 38(4):967–972
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430(7000):631–639
Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC (2004) Wnt-3a overcomes b-amyloid toxicity in rat hippocampal neurons. Exp Cell Res 297:186–196
Vargas JY, Fuenzalida M, Inestrosa NC (2014) In vivo activation of Wnt signalling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J Neurosci 34:2191–2202
Cerpa W, Toledo EM, Varela-Nallar L et al (2009) The role of Wnt signalling in neuroprotection. Drug News Perspect 22:579–591
Quintanilla RA, Muñoz FJ, Metcalfe MJ, Hitschfeld M, Olivares G, Godoy JA et al (2005) Trolox and 17beta-estradiol protect against amyloid beta-peptide neurotoxicity by a mechanism that involves modulation of the Wnt signalling pathway. J Biol Chem 280:11615–11625. https://doi.org/10.1074/jbc.M411936200
Silva-Alvarez C, Arrázola MS, Godoy JA, Ordenes D, Inestrosa NC (2013) Canonical Wnt signalling protects hippocampal neurons from A beta oligomers: role of non-canonical Wnt-5a/Ca(2+) in mitochondrial dynamics. Front Cell Neurosci 7:97. https://doi.org/10.3389/fncel.2013.00097
Arrazola MS, Varela-Nallar L, Colombres M, Toledo EM, Cruzat F, Pavez L et al (2009) Calcium/calmodulin-dependent protein kinase type IV is a target gene of the Wnt/beta-catenin signaling pathway. J Cell Physiol 221:658–667
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11:77–86
Varela-Nallar L, Inestrosa NC (2013) Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci 7:100
Inestrosa NC, Varela-Nallar L (2014) Wnt signalling in the nervous system and in Alzheimer’s disease. J Mol Cell Biol 6:64–74. https://doi.org/10.1093/jmcb/mjt051
Oliva CA, Inestrosa NC (2015) A novel function for Wnt signalling modulating neuronal firing activity and the temporal structure of spontaneous oscillation in the entorhinal-hippocampal circuit. Exp Neurol 269:43–55
De Ferrari GV, Chacon MA, Barria MI et al (2003) Activation of Wnt signalling rescues neurodegeneration and behavioral impairments induced by b-amyloid fibrils. Mol Psychiatry 8:195–208
Vargas JY, Fuenzalida M, Inestrosa NC (2014) In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J Neurosci 34(6):2191–2202
Husain I, Zameer S, Madaan T, Minhaj A, Ahmad W, Iqubaal A, Ali A, Najmi AK (2019) Exploring the multifaceted neuroprotective actions of Emblica officinalis (Amla): a review. Metab Brain Dis 8:1–9
Rosi MC, Luccarini I, Grossi C, Fiorentini A, Spillantini MG, Prisco A, Scali C, Gianfriddo M et al (2010) Increased dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. J Neurochem 112(6):1539–1551
Killick R, Ribe EM, Al-Shawi R, Malik B, Hooper C, Fernandes C et al (2014) Clusterin regulates beta-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol Psychiatry 19:88–98. https://doi.org/10.1038/mp.2012.163
Purro SA, Dickins EM, Salinas PC (2012) The secreted Wnt antagonist dickkopf-1 is required for amyloid 훽-mediated synaptic loss. J Neurosci 32(10):3492–3498
Chacon MA, Varela-Nallar L, Inestrosa NC (2008) Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Ab oligomers. J Cell Physiol 217:215–227
Wan W, Chen H, Li Y (2014) The potential mechanisms of Abeta-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer’s disease. Int J Neurosci 124(2):75–81
Kook SY, Hong HS, Moon M, Ha CM, Chang S, Mook-Jung I (2012) Abeta1−42-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca2+-calcineurin signaling. J Neurosci 32(26):8845–8854
Mendoza J, Sekiya M, Taniguchi T, Iijima KM, Wang R, Ando K (2013) Global analysis of phosphorylation of tau by the checkpoint kinases Chk1 and Chk2 in vitro. J Proteome Res 12(6):2654–2665
Folwell J, Cowan CM, Ubhi KK, Shiabh H, Newman TA, Shepherd D, Mudher A (2010) A훽 exacerbates the neuronal dysfunction caused by human tau expression in a Drosophila model of Alzheimer’s disease. Exp Neurol 223(2):401–409
Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC (2004) Wnt-3a overcomes 훽-amyloid toxicity in rat hippocampal neurons. Exp Cell Res 297(1):186–196
Maguschak KA, Ressler KJ (2012) A role for WNT/beta-catenin signaling in the neural mechanisms of behavior. J NeuroImmune Pharmacol 7(4):763–773
Shruster A, Eldar-Finkelman H, Melamed E, Offen D (2011) Wnt signaling pathway overcomes the disruption of neuronal differentiation of neural progenitor cells induced by oligomeric amyloid 훽-peptide. J Neurochem 116(4):522–529
Cisternas P, Salazar P, Silva-Álvarez C, Barros L (2016) F, Inestrosa NC. Activation of Wnt signaling in cortical neurons enhances glucose utilization through glycolysis. J Biol Chem 291(50):25950–25964
Schinner S (2009) Wnt-signalling and the metabolic syndrome. Horm Metab Res 41:159–163
Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17:491–506
Brown AM (2004) Brain glycogen re-awakened. J Neurochem 89:537–552
Saez I, Duran J, Sinadinos C, Beltran A, Yanes O, Tevy MF, Martínez-Pons C, Milán M et al (2014) Neurons have an active glycogen metabolism that contributes to tolerance to hypoxia. J Cereb Blood Flow Metab 34:945–955
Vilchez D, Ros S, Cifuentes D, Pujadas L, Vallès J, García-Fojeda B, Criado-García O, Fernández-Sánchez E et al (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10:1407–1413
Solaz-Fuster MC, Gimeño-Alcañiz JV, Ros S, Fernandez-Sanchez ME, Garcia-Fojeda B, Criado Garcia O et al (2008) Regulation of glycogen synthesis by the laforin-malin complex is modulated by the AMP activated protein kinase pathway. Hum Mol Genet 17:667–678
Cohen P, Goedert M (2004) GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov 3:479–487
Cadigan KM (2012) TCFs and Wnt/b-catenin signaling: more than one way ot throw the switch. Transcriptional Switches during Development. Curr Top Dev Biol 98:1–34
Godoy JA, Arrázola MS, Ordenes D, Silva-Alvarez C, Braidy N, Inestrosa NC (2014) Wnt-5a ligand modulates mitochondrial fission-fusion in rat hippocampal neurons. J Biol Chem 289:36179–36193
Chen EY, DeRan MT, Ignatius MS, Grandinetti KB, Clagg R, McCarthy KM et al (2014) Glycogen synthase kinase 3 inhibitors induce the canonical WNT/beta-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma. Proc Natl Acad Sci U S A 111:5349–5354
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, A., Ali, A., Ahmad, W. et al. Deciphering the Role of WNT Signaling in Metabolic Syndrome–Linked Alzheimer’s Disease. Mol Neurobiol 57, 302–314 (2020). https://doi.org/10.1007/s12035-019-01700-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01700-y