Abstract
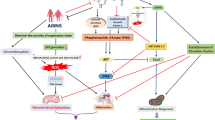
Melatonin, a pineal gland synthesized neurohormone is known as a multifunctioning pleiotropic agent which has a wide range of neuroprotective role in manifold age-related neurodegenerative disorders especially Alzheimer’s diseases (AD). AD is a devastating neurodegenerative disorder and common form of dementia which is defined by abnormal and excessive accumulation of several toxic peptides including amyloid β (Aβ) plaques and neurofibrillary tangles (NFTs). The Alzheimer’s dementia relates to atrophic changes in the brain resulting in loss of memory, cognitive dysfunction, and impairments of the synapses. Aging, circadian disruption, Aβ accumulation, and tau hyperphosphorylation are the utmost risk factor regarding AD pathology. To date, there is no exact treatment against AD progression. In this regard, melatonin plays a crucial role for the inhibition of circadian disruption by controlling clock genes and also attenuates Aβ accumulation and tau hyperphosphorylation by regulating glycogen synthase kinase-3 (GSK3) and cyclin-dependent kinase-5 (CDK5) signaling pathway. In this review, we highlight the possible mechanism of AD etiology and how melatonin influences neurogenesis by attenuating circadian disruption, Aβ formation, as well as tau hyperphosphorylation. Furthermore, we also find out and summarize the neuroprotective roles of melatonin by the blockage of Aβ production, Aβ oligomerization and fibrillation, tau hyperphosphorylation, synaptic dysfunction, oxidative stress, and neuronal death during AD progression.





Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
amyloid β
- APP:
-
amyloid protein precursor
- AANAT:
-
N-acetylserotonin by arylalkylamine N-acetyltransferase
- AVP:
-
arginine vasopressin
- ACh:
-
acetylcholine
- AChE:
-
acetylcholinesterase
- AGEs:
-
advanced glycation end products
- ADAM10:
-
a disintegrin and metalloproteinase domain-containing protein-10
- AAD:
-
aromatic amino acid decarboxylase
- BMAL1:
-
brain muscle ARNT-like 1
- CSF:
-
cerebrospinal fluid
- CRY:
-
cryptochrome
- CLOCK:
-
circadian locomotor output cycles kaput
- COX2:
-
cyclooxygenase-2
- ChAT:
-
choline acetyltransferase
- C1q:
-
complement 1q
- CuZnSOD:
-
copper-zinc superoxide dismutase
- CDK5:
-
cyclin-dependent kinase 5
- ER:
-
endoplasmic reticulum
- GPx:
-
glutathione peroxidase
- H2O2 :
-
hydrogen peroxide
- HIOMT:
-
hydroxyindole-O-methyltransferase
- IL1-β:
-
interleukin-1-β
- IL6:
-
interleukin-6
- KA:
-
kainic acid
- LPS:
-
lipopolysaccharide
- LTP:
-
long-term potentiation
- LTD:
-
long-term depression
- MCI:
-
mild cognitive impairment
- MnSOD:
-
manganese superoxide dismutase
- NFTs:
-
neurofibrillary tangles
- NO:
-
nitric oxide
- NOS2:
-
nitric oxide synthase 2
- NF-κB:
-
nuclear factor kappa beta
- PLC:
-
phospholipase C
- PKC:
-
protein kinase C
- PI3K:
-
phosphatidylinositol 3-kinase
- PER:
-
period circadian protein homologue
- PSEN1:
-
presenilin-1
- PSEN2:
-
presenilin-2
- ROS:
-
reactive oxygen species
- RHT:
-
retinohypothalamic tract
- SOD:
-
superoxide dismutase
- SCN:
-
suprachiasmatic nucleus
- SCG:
-
superior cervical ganglion
- SIRT1:
-
sirtuin 1
- TNF-α:
-
tumor necrosis factor-α
- VIP:
-
vasoactive intestinal peptide
- 5-HTP:
-
5-hydroxytryptophan
- Bcl2:
-
B cell lymphoma 2
- PP2A:
-
protein phosphatase 2A
- GSK3β:
-
glycogen synthase kinase 3 beta
- PKA:
-
protein kinase-A
- Bax:
-
BCL2 associated X
- Par-4:
-
prostate apoptosis response-4
- JNK:
-
c-JUN N-terminal kinase
- ERK:
-
extracellular signal-regulated kinase
- MAO-A:
-
monoamine oxidase A
- RAGE:
-
receptor for advanced glycation end products
- GFAP:
-
glial fibrillary acidic protein
- Iba1:
-
ionized calcium binding adaptor molecule 1
- APOE:
-
apolipoprotein E
- PARP1:
-
poly(ADP-ribose) polymerase-1
References
Shukla M, Govitrapong P, Boontem P et al (2017) Mechanisms of melatonin in alleviating Alzheimer’s disease. Curr Neuropharmacol 15:1010–1031. https://doi.org/10.2174/1570159X15666170313123454
Uddin MS, Al Mamun A, Asaduzzaman M et al (2018) Spectrum of disease and prescription pattern for outpatients with neurological disorders: an empirical pilot study in Bangladesh. Ann Neurosci 25:25–37. https://doi.org/10.1159/000481812
Uddin MS, Stachowiak A, Mamun AA et al (2018) Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci 10:1-18. https://doi.org/10.3389/fnagi.2018.00004
Uddin MS, Mamun AA, Hossain MS et al (2016) Exploring the effect of Phyllanthus emblica L. on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: promising natural gift for the mitigation of Alzheimer’s disease. Ann Neurosci 23:218–229. https://doi.org/10.1159/000449482
Uddin MS, Mamun AA, Labu ZK et al (2018) Autophagic dysfunction in Alzheimer’s disease: cellular and molecular mechanistic approaches to halt Alzheimer’s pathogenesis. J Cell Physiol 234(6):8094–8112. https://doi.org/10.1002/jcp.27588
Uddin MS, Al Mamun A, Kabir MT, et al (2018) Nootropic and anti-Alzheimer’s actions of medicinal plants: molecular insight into therapeutic potential to alleviate Alzheimer’s neuropathology. Mol Neurobiol 1–20. https://doi.org/10.1007/s12035-018-1420-2
Uddin MS, Mamun AA, Takeda S et al (2018) Analyzing the chance of developing dementia among geriatric people: a cross-sectional pilot study in Bangladesh. Psychogeriatrics. 19(2):87–94. https://doi.org/10.1111/psyg.12368
Uddin MS, Amran MS (eds) (2018) Handbook of research on critical examinations of neurodegenerative disorders, 1st ed. USA: IGI Global. https://doi.org/10.4018/978-1-5225-5282-6
Mamum AA, Uddin MS, Wahid F et al (2016) Neurodefensive effect of Olea europaea L. in alloxan-induced cognitive dysfunction and brain tissue oxidative stress in mice: incredible natural nootropic. J Neurol Neurosci 7:1-12. https://doi.org/10.21767/2171-6625.1000126
Uddin MS, Mamun AA, Iqbal MA et al (2016) Analyzing nootropic effect of Phyllanthus reticulatus Poir. on cognitive functions, brain antioxidant enzymes and acetylcholinesterase activity against aluminium-induced Alzheimer’s model in rats: applicable for controlling the risk factors of Alzheimer’s disease. Adv Alzheimer’s Dis 05:87–102. https://doi.org/10.4236/aad.2016.53007
Grandy JK (2013) Melatonin: therapeutic intervention in mild cognitive impairment and Alzheimer disease. J Neurol Neurophysiol 04:1–6. https://doi.org/10.4172/2155-9562.1000148
Uddin MS, Kabir MT, Al Mamun A et al (2018) APOE and Alzheimer’s disease: evidence mounts that targeting APOE4 may combat Alzheimer’s pathogenesis. Mol Neurobiol 56(4):2450–2465. 1–16. https://doi.org/10.1007/s12035-018-1237-z
ADI G8 (2050) Policy Briefing reveals 135 million people will live with dementia by Alzheimer’s Disease International
Savaskan E, Ayoub MA, Ravid R et al (2005) Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J Pineal Res 38:10–16. https://doi.org/10.1111/j.1600-079X.2004.00169.x
Naseem M, Parvez S (2014) Role of melatonin in traumatic brain injury and spinal cord injury. Sci World J 2014:1–13. https://doi.org/10.1155/2014/586270
Wang L, Feng C, Zheng X et al (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J Pineal Res 63:e12429. https://doi.org/10.1111/jpi.12429
Payne JK (2006) The trajectory of biomarkers in symptom management for older adults with cancer. Semin Oncol Nurs 22:31–35. https://doi.org/10.1016/j.soncn.2005.10.005
Sugden D (1983) Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther 227:587–591
Bilici D, Akpinar E, Kiziltunç A (2002) Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol Res 46:133–139
Carrillo-Vico A, Lardone P, Álvarez-Sánchez N et al (2013) Melatonin: buffering the immune system. Int J Mol Sci 14:8638–8683. https://doi.org/10.3390/ijms14048638
Srinivasan V, Pandi-Perumal SR, Spence DW et al (2010) Potential use of melatonergic drugs in analgesia: mechanisms of action. Brain Res Bull 81:362–371. https://doi.org/10.1016/j.brainresbull.2009.12.001
Tan D-X, Manchester LC, Sanchez-Barcelo E et al (2010) Significance of high levels of endogenous melatonin in mammalian cerebrospinal fluid and in the central nervous system. Curr Neuropharmacol 8:162–167. https://doi.org/10.2174/157015910792246182
Benot S, Molinero P, Soutto M et al (1998) Circadian variations in the rat serum total antioxidant status: correlation with melatonin levels. J Pineal Res 25:1–4. https://doi.org/10.1111/j.1600-079X.1998.tb00378.x
Kasahara T, Abe K, Mekada K et al (2010) Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci 107:6412–6417. https://doi.org/10.1073/pnas.0914399107
Semenova NV, Madaeva IM, Bairova TA et al (2018) Association of the melatonin circadian rhythms with clock 3111T/C gene polymorphism in Caucasian and Asian menopausal women with insomnia. Chronobiol Int 35:1–11. https://doi.org/10.1080/07420528.2018.1456447
Reiter RJ (1995) The pineal gland and melatonin in relation to aging: a summary of the theories and of the data. Exp Gerontol 30:199–212
Reiter RJ, Tan DX, Poeggeler B et al (1994) Melatonin as a free radical scavenger: implications for aging and age-related diseases. Ann N Y Acad Sci 719:1–12
Rosales-Corral S, Tan D-X, Reiter RJ et al (2003) Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-beta peptide in rat brain: a comparative, in vivo study versus vitamin C and E. J Pineal Res 35:80–84
Smith MA, Hirai K, Hsiao K et al (1998) Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem 70:2212–2215
Wu Y-H, Feenstra MGP, Zhou J-N et al (2003) Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab 88:5898–5906. https://doi.org/10.1210/jc.2003-030833
Wu Y-H, Swaab DF (2005) The human pineal gland and melatonin in aging and Alzheimer’s disease. J Pineal Res 38:145–152. https://doi.org/10.1111/j.1600-079X.2004.00196.x
Rosales-Corral SA, Acuña-Castroviejo D, Coto-Montes A et al (2012) Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res 52:167–202. https://doi.org/10.1111/j.1600-079X.2011.00937.x
Lin L, Huang Q-X, Yang S-S et al (2013) Melatonin in Alzheimer’s disease. Int J Mol Sci 14:14575–14593. https://doi.org/10.3390/ijms140714575
Devi L, Prabhu BM, Galati DF et al (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci 26:9057–9068. https://doi.org/10.1523/JNEUROSCI.1469-06.2006
Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med 7:548–554
Lahiri DK, Ghosh C (1999) Interactions between melatonin, reactive oxygen species, and nitric oxide. Ann N Y Acad Sci 893:325–330
Matsubara E, Bryant-Thomas T, Pacheco Quinto J et al (2003) Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem 85:1101–1108. https://doi.org/10.1046/j.1471-4159.2003.01654.x
Lahiri DK, Chen D, Ge Y-W et al (2004) Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J Pineal Res 36:224–231. https://doi.org/10.1111/j.1600-079X.2004.00121.x
Song W, Lahiri DK (1997) Melatonin alters the metabolism of the β-amyloid precursor protein in the neuroendocrine cell line PC12. J Mol Neurosci 9:75–92. https://doi.org/10.1007/BF02736852
Zhang Y, Wang Z, Wang Q et al (2004) Melatonin attenuates beta-amyloid-induced inhibition of neurofilament expression. Acta Pharmacol Sin 25:447–451
Olivieri G, Hess C, Savaskan E et al (2001) Melatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased beta-amyloid secretion. J Pineal Res 31:320–325
Zhu LQ, Wang SH, Ling ZQ et al (2004) Effect of inhibiting melatonin biosynthesis on spatial memory retention and tau phosphorylation in rat. J Pineal Res 37:71–77. https://doi.org/10.1111/j.1600-079X.2004.00136.x
Corpas R, Griñán-Ferré C, Palomera-Ávalos V et al (2018) Melatonin induces mechanisms of brain resilience against neurodegeneration. J Pineal Res 65:e12515. https://doi.org/10.1111/jpi.12515
Kang J-E, Lim MM, Bateman RJ et al (2009) Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science (80-) 326:1005–1007. https://doi.org/10.1126/science.1180962
Moe V, Larsen P (1995) Symposium: Cognitive processes and sleep disturbances: sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res 4:15–20
Brusco LI, Márquez M, Cardinali DP (1998) Monozygotic twins with Alzheimer’s disease treated with melatonin: case report. J Pineal Res 25:260–263
Brusco L, Fainstein I, Márquez M, Cardinali D (1999) Effect of melatonin in selected populations of sleep-disturbed patients. Neurosignals 8:126–131. https://doi.org/10.1159/000014580
Brusco LI, Márquez M, Cardinali DP (2000) Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer’s disease. Neuro Endocrinol Lett 21:39–42
Poeggeler B, Miravalle L, Zagorski MG et al (2001) Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry 40:14995–15001
Feng Z, Chang Y, Cheng Y et al (2004) Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J Pineal Res 37:129–136. https://doi.org/10.1111/j.1600-079X.2004.00144.x
Wang X-C, Zhang J, Yu X et al (2005) Prevention of isoproterenol-induced tau hyperphosphorylation by melatonin in the rat. Sheng Li Xue Bao 57:7–12
McCurry SM, Reynolds CF, Ancoli-Israel S et al (2000) Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev 4:603–628. https://doi.org/10.1053/smrv.2000.0127
Lerner AB, Case JD, Takahashi Y et al (1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes 1. J Am Chem Soc 80:2587–2587. https://doi.org/10.1021/ja01543a060
Ueck M, Wake K (1977) The pinealocyte--a paraneuron? A review Arch Histol Jpn 40(Suppl):261–278
Stehle JH, Saade A, Rawashdeh O et al (2011) A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res 51:17–43. https://doi.org/10.1111/j.1600-079X.2011.00856.x
Yonei Y, Hattori A, Tsutsui K, et al (2010) Effects of melatonin: basics studies and clinical applications
Stokkan K-A, Reiter RJ (1994) Melatonin rhythms in Arctic urban residents. J Pineal Res 16:33–36. https://doi.org/10.1111/j.1600-079X.1994.tb00079.x
Sayler A, Wolfson A (1969) Hydroxyindole-o-methyl transferase (HIOMT) activity in the Japanese quail in relation to sexual maturation and light. Neuroendocrinology 5:322–332. https://doi.org/10.1159/000121892
Pévet P (2002) Melatonin. Dialogues Clin Neurosci 4:57–72
Armstrong SM, Redman JR (1991) Melatonin: a chronobiotic with anti-aging properties? Med Hypotheses 34:300–309
Kunz D (2004) Chronobiotic protocol and circadian sleep propensity index: new tools for clinical routine and research on melatonin and sleep. Pharmacopsychiatry 37:139–146. https://doi.org/10.1055/s-2004-827167
Maestroni GJ, Conti A (1989) Beta-endorphin and dynorphin mimic the circadian immunoenhancing and anti-stress effects of melatonin. Int J Immunopharmacol 11:333–340
Benot S, Goberna R, Reiter RJ et al (1999) Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J Pineal Res 27:59–64
Yon J-H, Carter LB, Reiter RJ, Jevtovic-Todorovic V (2006) Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis 21:522–530. https://doi.org/10.1016/j.nbd.2005.08.011
Hardeland R (2013) Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. J Pineal Res 55:N/a. https://doi.org/10.1111/jpi.12090
Thomas JN, Smith-Sonneborn J (1997) Supplemental melatonin increases clonal lifespan in the protozoan Paramecium tetraurelia. J Pineal Res 23:123–130. https://doi.org/10.1111/j.1600-079X.1997.tb00344.x
Pierpaoli W, Dall’Ara A, Pedrinis E, Regelson W (1991) The pineal control of aging. The effects of melatonin and pineal grafting on the survival of older mice. Ann N Y Acad Sci 621:291–313
Bonilla E, Medina-Leendertz S, Díaz S (2002) Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp Gerontol 37:629–638
Masana MI, Dubocovich ML (2001) Melatonin receptor signaling: finding the path through the dark. Sci Signal 2001:pe39–pe39. https://doi.org/10.1126/stke.2001.107.pe39
Cristòfol R, Porquet D, Corpas R et al (2012) Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J Pineal Res 52:271–281. https://doi.org/10.1111/j.1600-079X.2011.00939.x
Chang H-M, Wu U-I, Lan C-T (2009) Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res 47:211–220. https://doi.org/10.1111/j.1600-079X.2009.00704.x
Wang J, Fivecoat H, Ho L et al (2010) The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer’s disease neuropathology. Biochim Biophys Acta, Proteins Proteomics 1804:1690–1694. https://doi.org/10.1016/j.bbapap.2009.11.015
Albani D, Polito L, Batelli S et al (2009) The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1-42) peptide. J Neurochem 110:1445–1456. https://doi.org/10.1111/j.1471-4159.2009.06228.x
Julien C, Tremblay C, Émond V et al (2009) Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol 68:48–58. https://doi.org/10.1097/NEN.0b013e3181922348
Qin W, Yang T, Ho L et al (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754. https://doi.org/10.1074/jbc.M602909200
Tippmann F, Hundt J, Schneider A et al (2009) Up-regulation of the α-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J 23:1643–1654. https://doi.org/10.1096/fj.08-121392
West RL, Lee JM, Maroun LE (1995) Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J Mol Neurosci 6:141–146. https://doi.org/10.1007/BF02736773
Ding H, Dolan PJ, Johnson GVW (2008) Histone deacetylase 6 interacts with the microtubule-associated protein tau. J Neurochem 106:2119–2130. https://doi.org/10.1111/j.1471-4159.2008.05564.x
Jenwitheesuk A, Nopparat C, Mukda S et al (2014) Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int J Mol Sci 15:16848–16884. https://doi.org/10.3390/ijms150916848
Van Someren EJ (2000) Circadian and sleep disturbances in the elderly. Exp Gerontol 35:1229–1237
Foley DJ, Monjan AA, Brown SL et al (1995) Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 18:425–432
Huang Y-L, Liu R-Y, Wang Q-S et al (2002) Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav 76:597–603
McCurry SM, Logsdon RG, Teri L et al (1999) Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J Geriatr Psychiatry Neurol 12:53–59. https://doi.org/10.1177/089198879901200203
Bonanni E, Maestri M, Tognoni G et al (2005) Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J Sleep Res 14:311–317. https://doi.org/10.1111/j.1365-2869.2005.00462.x
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941. https://doi.org/10.1038/nature00965
Haugh RM, Markesbery WR (1983) Hypothalamic astrocytoma. Syndrome of hyperphagia, obesity, and disturbances of behavior and endocrine and autonomic function. Arch Neurol 40:560–563
Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM (1996) A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci 16:5555–5565
Hofman MA, Swaab DF (1995) Influence of aging on the seasonal rhythm of the vasopressin-expressing neurons in the human suprachiasmatic nucleus. Neurobiol Aging 16:965–971. https://doi.org/10.1016/0197-4580(95)02016-0
Swaab DF, Roozendaal B, Ravid R et al (1987) Suprachiasmatic nucleus in aging, Alzheimer’s disease, transsexuality and Prader-Willi syndrome. Prog Brain Res 72:301–310
Zhou J-N, Swaab DF (1999) Activation and degeneration during aging: a morphometric study of the human hypothalamus. Microsc Res Tech 44:36–48. https://doi.org/10.1002/(SICI)1097-0029(19990101)44:1<36::AID-JEMT5>3.0.CO;2-F
Zhou JN, Hofman MA, Swaab DF VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging 16:571–576
Swaab DF, Fliers E, Partiman TS (1985) The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res 342:37–44
Swaab DF, Grundke-Iqbal I, Iqbal K et al (1992) Tau and ubiquitin in the human hypothalamus in aging and Alzheimer’s disease. Brain Res 590:239–249
Liu RY, Zhou JN, Hoogendijk WJ et al (2000) Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol 59:314–322
Lowrey PL, Takahashi JS (2000) Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet 34:533–562. https://doi.org/10.1146/annurev.genet.34.1.533
Yoo S-H, Yamazaki S, Lowrey PL et al (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci 101:5339–5346. https://doi.org/10.1073/pnas.0308709101
Steeves TDL, King DP, Zhao Y et al (1999) Molecular cloning and characterization of the HumanCLOCKGene: expression in the suprachiasmatic nuclei. Genomics 57:189–200. https://doi.org/10.1006/geno.1998.5675
Simonneaux V, Poirel V-J, Garidou M-L et al (2004) Daily rhythm and regulation of clock gene expression in the rat pineal gland. Brain Res Mol Brain Res 120:164–172
Kolker DE, Fukuyama H, Huang DS et al (2003) Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythm 18:159–169. https://doi.org/10.1177/0748730403251802
Yamazaki S, Straume M, Tei H et al (2002) Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci 99:10801–10806. https://doi.org/10.1073/pnas.152318499
Claustrat B, Brun J, Chazot G (2005) The basic physiology and pathophysiology of melatonin. Sleep Med Rev 9:11–24. https://doi.org/10.1016/j.smrv.2004.08.001
Wu Y-H, Fischer DF, Kalsbeek A et al (2006) Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J 20:1874–1876. https://doi.org/10.1096/fj.05-4446fje
Song H, Moon M, Choe HK et al (2015) Aβ-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener 10:13. https://doi.org/10.1186/s13024-015-0007-x
Reppert SM, Weaver DR, Ebisawa T (1994) Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13:1177–1185
Ekmekcioglu C (2006) Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 60:97–108. https://doi.org/10.1016/j.biopha.2006.01.002
Weaver DR, Reppert SM (1996) The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport 8:109–112
Thomas L, Purvis CC, Drew JE et al (2002) Melatonin receptors in human fetal brain: 2-[(125)I]iodomelatonin binding and MT1 gene expression. J Pineal Res 33:218–224
Wu Y-H, Zhou J-N, Balesar R et al (2006) Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J Comp Neurol 499:897–910. https://doi.org/10.1002/cne.21152
Wu Y-H, Zhou J-N, Van Heerikhuize J et al (2007) Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging 28:1239–1247. https://doi.org/10.1016/j.neurobiolaging.2006.06.002
Liu C, Weaver DR, Jin X et al (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19:91–102
Zhou J-N, Liu R-Y, Kamphorst W et al (2003) Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res 35:125–130
Liu R-Y, Zhou J-N, van Heerikhuize J et al (1999) Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-ε4/4 genotype 1. J Clin Endocrinol Metab 84:323–327. https://doi.org/10.1210/jcem.84.1.5394
Reiter RJ, Tan DX, Manchester LC, El-Sawi MR (2002) Melatonin reduces oxidant damage and promotes mitochondrial respiration: implications for aging. Ann N Y Acad Sci 959:238–250
Reiter RJ, Tan D, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress. J Biomed Sci 7:444–458. https://doi.org/10.1159/000025480
Pappolla MA, Chyan Y-J, Poeggeler B et al (2000) An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer’s disease. J Neural Transm 107:203–231. https://doi.org/10.1007/s007020050018
Wang J, Wang Z (2006) Role of melatonin in Alzheimer-like neurodegeneration1. Acta Pharmacol Sin 27:41–49. https://doi.org/10.1111/j.1745-7254.2006.00260.x
Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI (2003) Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci 8:d1093–d1108
Reppert SM, Godson C, Mahle CD et al (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc Natl Acad Sci 92:8734–8738. https://doi.org/10.1073/pnas.92.19.8734
Lockley SW, Skene DJ, James K et al (2000) Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol 164:R1–R6
Sack RL, Brandes RW, Kendall AR, Lewy AJ (2000) Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med 343:1070–1077. https://doi.org/10.1056/NEJM200010123431503
Skene DJ (2003) Optimization of light and melatonin to phase-shift human circadian rhythms. J Neuroendocrinol 15:438–441
Swaab DF (Dick F (2003) The human hypothalamus : basic and clinical aspects. Elsevier
Arendt J, Skene DJ, Middleton B et al (1997) Efficacy of melatonin treatment in jet lag, shift work, and blindness. J Biol Rhythm 12:604–617. https://doi.org/10.1177/074873049701200616
Duffy JF, Zeitzer JM, Czeisler CA (2007) Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging 28:799–807. https://doi.org/10.1016/j.neurobiolaging.2006.03.005
Herljevic M, Middleton B, Thapan K, Skene D (2005) Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol 40:237–242. https://doi.org/10.1016/j.exger.2004.12.001
Shochat T, Martin J, Marler M, Ancoli-Israel S (2000) Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res 9:373–379
Mishima K, Okawa M, Shimizu T, Hishikawa Y (2001) Diminished melatonin secretion in the elderly caused by insufficient environmental illumination 1. J Clin Endocrinol Metab 86:129–134. https://doi.org/10.1210/jcem.86.1.7097
Ancoli-Israel S, Klauber MR, Jones DW et al (1997) Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep 20:18–23
Ohashi Y, Okamoto N, Uchida K et al (1999) Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol Psychiatry 45:1646–1652
Laurin D, Verreault R, Lindsay J et al (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 58:498–504
Barnes LL, Mendes de Leon CF, Wilson RS et al (2004) Social resources and cognitive decline in a population of older African Americans and whites. Neurology 63:2322–2326
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8:101–112. https://doi.org/10.1038/nrm2101
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science (80- ) 298:789–791. https://doi.org/10.1126/science.1074069
He H, Dong W, Huang F (2010) Anti-amyloidogenic and anti-apoptotic role of melatonin in Alzheimer disease. Curr Neuropharmacol 8:211–217. https://doi.org/10.2174/157015910792246137
Wang X-C, Zhang Y-C, Chatterjie N et al (2008) Effect of melatonin and melatonylvalpromide on β-amyloid and neurofilaments in N2a cells. Neurochem Res 33:1138–1144. https://doi.org/10.1007/s11064-007-9563-y
Jin N, Yin X, Yu D et al (2015) Truncation and activation of GSK-3β by calpain I: a molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci Rep 5:8187. https://doi.org/10.1038/srep08187
van Eersel J, Ke YD, Liu X et al (2010) Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc Natl Acad Sci 107:13888–13893. https://doi.org/10.1073/pnas.1009038107
Shimohama S, Tanino H, Fujimoto S (1999) Changes in caspase expression in Alzheimer’s disease: comparison with development and aging. Biochem Biophys Res Commun 256:381–384. https://doi.org/10.1006/bbrc.1999.0344
Tesco G, Koh YH, Kang EL et al (2007) Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 54:721–737. https://doi.org/10.1016/j.neuron.2007.05.012
Oh S, Gwak J, Park S, Yang CS (2014) Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. BioFactors 40:586–595. https://doi.org/10.1002/biof.1185
Yang C-C, Kuai X-X, Li Y-L et al (2013) Cornel iridoid glycoside attenuates tau hyperphosphorylation by inhibition of PP2A demethylation. Evid Based Complement Alternat Med 2013:108486. https://doi.org/10.1155/2013/108486
Louneva N, Cohen JW, Han L-Y et al (2008) Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am J Pathol 173:1488–1495. https://doi.org/10.2353/ajpath.2008.080434
Espino J, Bejarano I, Redondo PC et al (2010) Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: evidence for the involvement of mitochondria and Bax activation. J Membr Biol 233:105–118. https://doi.org/10.1007/s00232-010-9230-0
Ling X, Zhang LM, Lu SD et al (1999) Protective effect of melatonin on injuried cerebral neurons is associated with bcl-2 protein over-expression. Zhongguo Yao Li Xue Bao 20:409–414
Jang M-H, Jung S-B, Lee M-H et al (2005) Melatonin attenuates amyloid beta25–35-induced apoptosis in mouse microglial BV2 cells. Neurosci Lett 380:26–31. https://doi.org/10.1016/j.neulet.2005.01.003
Harada J, Sugimoto M (1999) Activation of caspase-3 in beta-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res 842:311–323
Sahab Uddin M, Nasrullah M, Hossain MS et al (2016) Evaluation of nootropic activity of Persicaria flaccida on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: implication for the management of Alzheimer’s disease. Am J Psychiatry Neurosci 4:26. https://doi.org/10.11648/j.ajpn.20160402.12
Uddin MS, Asaduzzaman M, Mamun AA, Iqbal MA, Wahid FRR (2016) Neuroprotective activity of Asparagus racemosus Linn. Against ethanol- induced cognitive impairment and oxidative stress in rats brain: auspicious for controlling the risk of Alzheimer’s disease. J Alzheimer’s Dis Park 6:1–10. https://doi.org/10.4172/2161-0460.1000245
Gong Y-H, Hua N, Zang X et al (2018) Melatonin ameliorates Aβ 1-42-induced Alzheimer’s cognitive deficits in mouse model. J Pharm Pharmacol 70:70–80. https://doi.org/10.1111/jphp.12830
O’Neal-Moffitt G, Delic V, Bradshaw PC, Olcese J (2015) Prophylactic melatonin significantly reduces Alzheimer’s neuropathology and associated cognitive deficits independent of antioxidant pathways in AβPPswe/PS1 mice. Mol Neurodegener 10:27. https://doi.org/10.1186/s13024-015-0027-6
Cleveland DW, Hwo SY, Kirschner MW (1977) Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol 116:227–247
Shipton OA, Leitz JR, Dworzak J et al (2011) Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J Neurosci 31:1688–1692. https://doi.org/10.1523/JNEUROSCI.2610-10.2011
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Ferrer I, Gomez-Isla T, Puig B et al (2005) Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res 2:3–18
Moreira PI, Honda K, Zhu X et al (2006) Brain and brawn: parallels in oxidative strength. Neurology 66:S97–S101. https://doi.org/10.1212/01.wnl.0000192307.15103.83
Lee VM, Balin BJ, Otvos L, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science 251:675–678
Khatoon S, Grundke-Iqbal I, Iqbal K (1992) Brain levels of microtubule-associated protein tau are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem 59:750–753
Khatoon S, Grundke-Iqbal I, Iqbal K (1994) Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett 351:80–84
Costa EJ, Lopes RH, Lamy-Freund MT (1995) Permeability of pure lipid bilayers to melatonin. J Pineal Res 19:123–126
Deng Y, Xu G, Duan P et al (2005) Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol Sin 26:519–526. https://doi.org/10.1111/j.1745-7254.2005.00102.x
Pandi-Perumal SR, Cardinali DP (2007) Melatonin : from molecules to therapy. Nova Biomedical Books
Peng C-X, Hu J, Liu D et al (2013) Disease-modified glycogen synthase kinase-3β intervention by melatonin arrests the pathology and memory deficits in an Alzheimer’s animal model. Neurobiol Aging 34:1555–1563. https://doi.org/10.1016/j.neurobiolaging.2012.12.010
Feng Z, Qin C, Chang Y, Zhang J (2006) Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of Alzheimer’s disease. Free Radic Biol Med 40:101–109. https://doi.org/10.1016/j.freeradbiomed.2005.08.014
Bhat RV, Budd Haeberlein SL, Avila J (2004) Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem 89:1313–1317. https://doi.org/10.1111/j.1471-4159.2004.02422.x
Eldar-Finkelman H (2002) Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med 8:126–132
Li X, Bijur GN, Jope RS (2002) Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord 4:137–144
Bhat RV, Budd SL (2002) GSK3beta signalling: casting a wide net in Alzheimer’s disease. Neurosignals 11:251–261. https://doi.org/10.1159/000067423
Krasilnikov MA (2000) Phosphatidylinositol-3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry (Mosc) 65:59–67
Hoppe JB, Frozza RL, Horn AP et al (2010) Amyloid-β neurotoxicity in organotypic culture is attenuated by melatonin: involvement of GSK-3β, tau and neuroinflammation. J Pineal Res 48:230–238. https://doi.org/10.1111/j.1600-079X.2010.00747.x
Wang J, Xiao X, Zhang Y et al (2012) Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res 53:77–90. https://doi.org/10.1111/j.1600-079X.2012.00973.x
Xue F, Shi C, Chen Q et al (2017) Melatonin mediates protective effects against kainic acid-induced neuronal death through safeguarding ER stress and mitochondrial disturbance. Front Mol Neurosci 10:49. https://doi.org/10.3389/fnmol.2017.00049
Shi C, Zeng J, Li Z et al (2018) Melatonin mitigates kainic acid-induced neuronal tau hyperphosphorylation and memory deficits through alleviating ER stress. Front Mol Neurosci 11(5). https://doi.org/10.3389/fnmol.2018.00005
Medeiros R, Baglietto-Vargas D, LaFerla FM (2011) The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci Ther 17:514–524. https://doi.org/10.1111/j.1755-5949.2010.00177.x
Salminen A, Kauppinen A, Suuronen T et al (2009) ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation 6:41. https://doi.org/10.1186/1742-2094-6-41
Hoozemans JJM, Veerhuis R, Van Haastert ES et al (2005) The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol 110:165–172. https://doi.org/10.1007/s00401-005-1038-0
Kim JS, Heo RW, Kim H et al (2014) Salubrinal, ER stress inhibitor, attenuates kainic acid-induced hippocampal cell death. J Neural Transm 121:1233–1243. https://doi.org/10.1007/s00702-014-1208-0
Zhang X-M, Zhu J (2011) Kainic acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Curr Neuropharmacol 9:388–398. https://doi.org/10.2174/157015911795596540
Fernández-Bachiller MI, Pérez C, Campillo NE et al (2009) Tacrine-melatonin hybrids as multifunctional agents for Alzheimer’s disease, with cholinergic, antioxidant, and neuroprotective properties. ChemMedChem 4:828–841. https://doi.org/10.1002/cmdc.200800414
Spuch C, Antequera D, Isabel Fernandez-Bachiller M et al (2010) A new Tacrine–melatonin hybrid reduces amyloid burden and behavioral deficits in a mouse model of Alzheimer’s disease. Neurotox Res 17:421–431. https://doi.org/10.1007/s12640-009-9121-2
Lau WWI, Ng JKY, Lee MMK et al (2012) Interleukin-6 autocrine signaling mediates melatonin MT 1/2 receptor-induced STAT3 Tyr 705 phosphorylation. J Pineal Res 52:477–489. https://doi.org/10.1111/j.1600-079X.2011.00965.x
Shen Y, Zhang G, Liu L, Xu S (2007) Suppressive effects of melatonin on amyloid-β-induced glial activation in rat hippocampus. Arch Med Res 38:284–290. https://doi.org/10.1016/j.arcmed.2006.10.007
Paouri E, Tzara O, Kartalou G-I et al (2017) Peripheral tumor necrosis factor-alpha (TNF-α) modulates amyloid pathology by regulating blood-derived immune cells and glial response in the brain of AD/TNF transgenic mice. J Neurosci 37:5155–5171. https://doi.org/10.1523/JNEUROSCI.2484-16.2017
McGeer PL, McGeer EG (1995) The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev 21:195–218
Uddin MS, Kabir MT, Jakaria M, et al (2019) Endothelial PPARγ is crucial for averting age-related vascular dysfunction by stalling oxidative stress and ROCK. Neurotox Res https://doi.org/10.1007/s12640-019-00047-5
Begum MM, Islam A, Begum R et al (2019) Ethnopharmacological inspections of organic extract of Oroxylum indicum in rat models: a promising natural gift. Evidence-Based Complement Altern Med 2019:1–13. https://doi.org/10.1155/2019/1562038
Hüll M, Berger M, Volk B, Bauer J (1996) Occurrence of interleukin-6 in cortical plaques of Alzheimer’s disease patients may precede transformation of diffuse into neuritic plaques. Ann N Y Acad Sci 777:205–212
Campbell IL, Abraham CR, Masliah E et al (1993) Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A 90:10061–10065
Strauss S, Bauer J, Ganter U et al (1992) Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab Investig 66:223–230
Selkoe DJ (1996) Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem 271:18295–18298
Zhang L, Zhao B, Yew DT et al (1997) Processing of Alzheimer’s amyloid precursor protein during H2O2-induced apoptosis in human neuronal cells. Biochem Biophys Res Commun 235:845–848. https://doi.org/10.1006/BBRC.1997.6698
Hsiao K, Chapman P, Nilsen S et al (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Liu X, Xu Y, Chen S et al (2014) Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic Biol Med 68:234–246. https://doi.org/10.1016/j.freeradbiomed.2013.12.012
Anuthakoengkun A, Itharat A (2014) Inhibitory effect on nitric oxide production and free radical scavenging activity of Thai medicinal plants in osteoarthritic knee treatment. J Med Assoc Thail 97(Suppl 8):S116–S124
Guenther AL, Schmidt SI, Laatsch H et al (2005) Reactions of the melatonin metabolite AMK (N1-acetyl-5-methoxykynuramine) with reactive nitrogen species: formation of novel compounds, 3-acetamidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J Pineal Res 39:251–260. https://doi.org/10.1111/j.1600-079X.2005.00242.x
Tan D, Reiter RJ, Manchester LC et al (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2:181–197
Antolín I, Rodríguez C, Saínz RM et al (1996) Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J 10:882–890
Shen YX, Xu SY, Wei W et al (2002) The protective effects of melatonin from oxidative damage induced by amyloid beta-peptide 25-35 in middle-aged rats. J Pineal Res 32:85–89
Shen Y-X, Xu S-Y, Wei W et al (2002) Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J Pineal Res 32:173–178
Wakatsuki A, Okatani Y, Shinohara K et al (2001) Melatonin protects fetal rat brain against oxidative mitochondrial damage. J Pineal Res 30:22–28
Smith MA, Sayre LM, Monnier VM, Perry G (1995) Radical AGEing in Alzheimer’s disease. Trends Neurosci 18:172–176
Uddin MS, Mamun AA, Kabir MT, et al (2017) Neurochemistry of neurochemicals: messengers of brain functions. Journal of Intellectual Disability - Diagnosis and Treatment 5:137-151. https://doi.org/10.6000/2292-2598.2017.05.04.6
Mecocci P, MacGarvey U, Beal MF (1994) Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol 36:747–751. https://doi.org/10.1002/ana.410360510
Weldon DT, Rogers SD, Ghilardi JR et al (1998) Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J Neurosci 18:2161–2173
Du Yan S, Zhu H, Fu J et al (1997) Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci U S A 94:5296–5301
Yan SD, Roher A, Chaney M et al (2000) Cellular cofactors potentiating induction of stress and cytotoxicity by amyloid beta-peptide. Biochim Biophys Acta 1502:145–157
Perini G, Della-Bianca V, Politi V et al (2002) Role of p75 neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines. J Exp Med 195:907–918
Walker DG, Lue L-F, Beach TG (2002) Increased expression of the urokinase plasminogen-activator receptor in amyloid beta peptide-treated human brain microglia and in AD brains. Brain Res 926:69–79
Brandt R, Hundelt M, Shahani N (2005) Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta Mol basis Dis 1739:331–354. https://doi.org/10.1016/j.bbadis.2004.06.018
Takuma K, Yan SS, Stern DM, Yamada K (2005) Mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in Alzheimer’s disease. J Pharmacol Sci 97:312–316
Guermonprez L, Ducrocq C, Gaudry-Talarmain YM (2001) Inhibition of acetylcholine synthesis and tyrosine nitration induced by peroxynitrite are differentially prevented by antioxidants. Mol Pharmacol 60:838–846
Hensley K, Carney JM, Mattson MP et al (1994) A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A 91:3270–3274
Cardoso SM, Santana I, Swerdlow RH, Oliveira CR (2004) Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Aβ toxicity. J Neurochem 89:1417–1426. https://doi.org/10.1111/j.1471-4159.2004.02438.x
Sheehan JP, Swerdlow RH, Miller SW et al (1997) Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. J Neurosci 17:4612–4622
Swerdlow RH, Parks JK, Cassarino DS et al (1997) Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology 49:918–925
Beal MF (1998) Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta 1366:211–223
Beal MF (2000) Oxidative metabolism. Ann N Y Acad Sci 924:164–169
Thiffault C, Bennett JP (2005) Cyclical mitochondrial deltapsiM fluctuations linked to electron transport, F0F1 ATP-synthase and mitochondrial Na+/ca+2 exchange are reduced in Alzheimer’s disease cybrids. Mitochondrion 5:109–119. https://doi.org/10.1016/j.mito.2004.12.002
Trimmer PA, Keeney PM, Borland MK et al (2004) Mitochondrial abnormalities in cybrid cell models of sporadic Alzheimer’s disease worsen with passage in culture. Neurobiol Dis 15:29–39
Haughey NJ, Liu D, Nath A et al (2002) Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid β-peptide by amyloid β-peptide. NeuroMolecular Med 1:125–136. https://doi.org/10.1385/NMM:1:2:125
Donovan MH, Yazdani U, Norris RD et al (2006) Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol 495:70–83. https://doi.org/10.1002/cne.20840
Wen PH, Hof PR, Chen X et al (2004) The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol 188:224–237. https://doi.org/10.1016/j.expneurol.2004.04.002
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85. https://doi.org/10.1186/1750-1326-6-85
Hamilton A, Holscher C (2012) The effect of ageing on neurogenesis and oxidative stress in the APPswe/PS1deltaE9 mouse model of Alzheimer’s disease. Brain Res 1449:83–93. https://doi.org/10.1016/j.brainres.2012.02.015
Rodríguez JJ, Jones VC, Tabuchi M et al (2008) Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One 3:e2935. https://doi.org/10.1371/journal.pone.0002935
Martinez-Canabal A (2014) Reconsidering hippocampal neurogenesis in Alzheimer’s disease. Front Neurosci 8:147. https://doi.org/10.3389/fnins.2014.00147
Haughey NJ, Nath A, Chan SL et al (2002) Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem 83:1509–1524
Ghosal K, Stathopoulos A, Pimplikar SW (2010) APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS One 5:e11866. https://doi.org/10.1371/journal.pone.0011866
Porayette P, Gallego MJ, Kaltcheva MM et al (2009) Differential processing of amyloid-β precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J Biol Chem 284:23806–23817. https://doi.org/10.1074/jbc.M109.026328
Phiel CJ, Wilson CA, Lee VM-Y, Klein PS (2003) GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 423:435–439. https://doi.org/10.1038/nature01640
Zhou F, Zhang L, Wang A et al (2008) The association of GSK3β with E2F1 facilitates nerve growth factor-induced neural cell differentiation. J Biol Chem 283:14506–14515. https://doi.org/10.1074/jbc.M706136200
Lie D-C, Colamarino SA, Song H-J et al (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437:1370–1375. https://doi.org/10.1038/nature04108
Anderton BH, Dayanandan R, Killick R, Lovestone S (2000) Does dysregulation of the notch and wingless/Wnt pathways underlie the pathogenesis of Alzheimer’s disease? Mol Med Today 6:54–59
Alvarez AR, Godoy JA, Mullendorff K et al (2004) Wnt-3a overcomes β-amyloid toxicity in rat hippocampal neurons. Exp Cell Res 297:186–196. https://doi.org/10.1016/j.yexcr.2004.02.028
Ramírez-Rodríguez G, Vega-Rivera NM, Benítez-King G et al (2012) Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci Lett 530:53–58. https://doi.org/10.1016/j.neulet.2012.09.045
Lacoste B, Angeloni D, Dominguez-Lopez S et al (2015) Anatomical and cellular localization of melatonin MT 1 and MT 2 receptors in the adult rat brain. J Pineal Res 58:397–417. https://doi.org/10.1111/jpi.12224
Domínguez-Alonso A, Ramírez-Rodríguez G, Benítez-King G (2012) Melatonin increases dendritogenesis in the hilus of hippocampal organotypic cultures. J Pineal Res 52:427–436. https://doi.org/10.1111/j.1600-079X.2011.00957.x
Liu J, Somera-Molina KC, Hudson RL, Dubocovich ML (2013) Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J Pineal Res 54:222–231. https://doi.org/10.1111/jpi.12023
Hong Y, Palaksha KJ, Park K et al (2010) REVIEW ARTICLE: Melatonin plus exercise-based neurorehabilitative therapy for spinal cord injury. J Pineal Res 49:201–209. https://doi.org/10.1111/j.1600-079X.2010.00786.x
Moriya T, Horie N, Mitome M, Shinohara K (2007) Melatonin influences the proliferative and differentiative activity of neural stem cells. J Pineal Res 42:411–418. https://doi.org/10.1111/j.1600-079X.2007.00435.x
Ramírez-Rodríguez G, Klempin F, Babu H et al (2009) Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 34:2180–2191. https://doi.org/10.1038/npp.2009.46
De Butte M, Pappas BA (2007) Pinealectomy causes hippocampal CA1 and CA3 cell loss: reversal by melatonin supplementation. Neurobiol Aging 28:306–313. https://doi.org/10.1016/j.neurobiolaging.2005.12.004
Rennie K, De Butte M, Pappas BA (2009) Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J Pineal Res 47:313–317. https://doi.org/10.1111/j.1600-079X.2009.00716.x
Tocharus C, Puriboriboon Y, Junmanee T et al (2014) Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience 275:314–321. https://doi.org/10.1016/j.neuroscience.2014.06.026
Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P (2010) Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J Pineal Res 49:291–300. https://doi.org/10.1111/j.1600-079X.2010.00794.x
McArthur AJ, Hunt AE, Gillette MU (1997) Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and Dawn 1. Endocrinology 138:627–634. https://doi.org/10.1210/endo.138.2.4925
Shukla M, Htoo HH, Wintachai P et al (2015) Melatonin stimulates the nonamyloidogenic processing of β APP through the positive transcriptional regulation of ADAM10 and ADAM17. J Pineal Res 58:151–165. https://doi.org/10.1111/jpi.12200
Agrawal R, Tyagi E, Shukla R, Nath C (2008) Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice. Behav Brain Res 189:381–386. https://doi.org/10.1016/j.bbr.2008.01.015
Ishida A, Furukawa K, Keller JN, Mattson MP (1997) Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport 8:2133–2137
Yuan T-F, Gu S, Shan C et al (2015) Oxidative stress and adult neurogenesis. Stem Cell Rev Rep 11:706–709. https://doi.org/10.1007/s12015-015-9603-y
Sarlak G, Jenwitheesuk A, Chetsawang B, Govitrapong P (2013) Effects of melatonin on nervous system aging: neurogenesis and neurodegeneration. J Pharmacol Sci 123:9–24
Uchida K, Okamoto N, Ohara K, Morita Y (1996) Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res 717:154–159
Skene DJ, Vivien-Roels B, Sparks DL et al (1990) Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Res 528:170–174
Mishima K, Tozawa T, Satoh K et al (1999) Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep–waking. Biol Psychiatry 45:417–421. https://doi.org/10.1016/S0006-3223(97)00510-6
Skene DJ, Swaab DF Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol 38:199–206
Reiter RJ, Tan D-X (2002) Role of CSF in the transport of melatonin. J Pineal Res 33:61
Reiter RJ (1993) The melatonin rhythm: both a clock and a calendar. Experientia 49:654–664
Cardinali DP, Brusco LI, Liberczuk C, Furio AM (2002) The use of melatonin in Alzheimer’s disease. Neuro Endocrinol Lett 23(Suppl 1):20–23
Cardinali DP, Furio AM, Brusco LI (2010) Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol 8:218–227. https://doi.org/10.2174/157015910792246209
Magri F, Locatelli M, Balza G et al (1997) Changes in endocrine orcadian rhythms as markers of physiological and pathological brain aging. Chronobiol Int 14:385–396. https://doi.org/10.3109/07420529709001459
Mahlberg R, Kunz D, Sutej I et al (2004) Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer disease: an open-label pilot study using actigraphy. J Clin Psychopharmacol 24:456–459
Asayama K, Yamadera H, Ito T et al (2003) Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J Nippon Med Sch 70:334–341
Hardeland R (2005) Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 27:119–130
Gunasingh Masilamoni J, Philip Jesudason E, Dhandayuthapani S et al (2008) The neuroprotective role of melatonin against amyloid β peptide injected mice. Free Radic Res 42:661–673. https://doi.org/10.1080/10715760802277388
Husson I, Mesplès B, Bac P et al (2002) Melatoninergic neuroprotection of the murine periventricular white matter against neonatal excitotoxic challenge. Ann Neurol 51:82–92
Zhu G, Wang D, Lin Y-H et al (2001) Protein kinase C ϵ suppresses Aβ production and promotes activation of α-secretase. Biochem Biophys Res Commun 285:997–1006. https://doi.org/10.1006/bbrc.2001.5273
Luo Y, Packer L (2006) Oxidative stress and age-related neurodegeneration. CRC/Taylor & Francis
Pappolla MA, Sos M, Omar RA et al (1997) Melatonin prevents death of neuroblastoma cells exposed to the {Alzheimer} amyloid peptide. J Neurosci 17:1683–1690
Guerrero-Muñoz MJ, Castillo-Carranza DL, Kayed R (2014) Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol 88:468–478. https://doi.org/10.1016/j.bcp.2013.12.023
Sengupta U, Nilson AN, Kayed R (2016) The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 6:42–49. https://doi.org/10.1016/j.ebiom.2016.03.035
Walsh DM, Tseng BP, Rydel RE et al (2000) The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry 39:10831–10839
Glabe CG (2006) Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging 27:570–575. https://doi.org/10.1016/j.neurobiolaging.2005.04.017
Chen Y-R, Glabe CG (2006) Distinct early folding and aggregation properties of Alzheimer amyloid-β peptides Aβ40 and Aβ42. J Biol Chem 281:24414–24422. https://doi.org/10.1074/jbc.M602363200
Stys PK, You H, Zamponi GW (2012) Copper-dependent regulation of NMDA receptors by cellular prion protein: Implications for neurodegenerative disorders. J Physiol 590:1357–1368. https://doi.org/10.1113/jphysiol.2011.225276
Gu Z, Liu W, Yan Z (2009) β-Amyloid impairs AMPA receptor trafficking and function by reducing ca 2+ /calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem 284:10639–10649. https://doi.org/10.1074/jbc.M806508200
Pappolla M, Bozner P, Soto C et al (1998) Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J Biol Chem 273:7185–7188
Poirier J, Davignon J, Bouthillier D et al (1993) Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet (London, England) 342:697–699
Bazoti FN, Tsarbopoulos A, Markides KE, Bergquist J (2005) Study of the non-covalent interaction between amyloid-β-peptide and melatonin using electrospray ionization mass spectrometry. J Mass Spectrom 40:182–192. https://doi.org/10.1002/jms.738
Johnstone M, Gearing AJ, Miller KM (1999) A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol 93:182–193
Scheff SW, Price DA (2003) Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging 24:1029–1046
Li S, Hong S, Shepardson NE et al (2009) Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62:788–801. https://doi.org/10.1016/j.neuron.2009.05.012
Palop JJ, Mucke L (2010) Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 13:812–818. https://doi.org/10.1038/nn.2583
Liu D, Yang Q, Li S (2013) Activation of extrasynaptic NMDA receptors induces LTD in rat hippocampal CA1 neurons. Brain Res Bull 93:10–16. https://doi.org/10.1016/j.brainresbull.2012.12.003
Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384. https://doi.org/10.1016/j.neurobiolaging.2005.09.012
Cardinali DP, Golombek DA, Rosenstein RE et al (1997) Melatonin site and mechanism of action: single or multiple? J Pineal Res 23:32–39. https://doi.org/10.1111/j.1600-079X.1997.tb00332.x
Corrales A, Vidal R, García S et al (2014) Chronic melatonin treatment rescues electrophysiological and neuromorphological deficits in a mouse model of Down syndrome. J Pineal Res 56:51–61. https://doi.org/10.1111/jpi.12097
Thiel G (1993) Synapsin I, synapsin II, and synaptophysin: marker proteins of synaptic vesicles. Brain Pathol 3:87–95
Ikeno T, Nelson RJ (2015) Acute melatonin treatment alters dendritic morphology and circadian clock gene expression in the hippocampus of Siberian hamsters. Hippocampus 25:142–148. https://doi.org/10.1002/hipo.22358
Rudnitskaya EA, Muraleva NA, Maksimova KY et al (2015) Melatonin attenuates memory impairment, amyloid-β accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. J Alzheimers Dis 47:103–116. https://doi.org/10.3233/JAD-150161
Benleulmi-Chaachoua A, Chen L, Sokolina K et al (2016) Protein interactome mining defines melatonin MT 1 receptors as integral component of presynaptic protein complexes of neurons. J Pineal Res 60:95–108. https://doi.org/10.1111/jpi.12294
Liu D, Wei N, Man H-Y et al (2015) The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ 22:583–596. https://doi.org/10.1038/cdd.2014.195
Ali T, Badshah H, Kim TH, Kim MO (2015) Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF- K B/JNK signaling pathway in aging mouse model. J Pineal Res 58:71–85. https://doi.org/10.1111/jpi.12194
Yoo DY, Kim W, Lee CH et al (2012) Melatonin improves d-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res 52:21–28. https://doi.org/10.1111/j.1600-079X.2011.00912.x
Olanow CW (1993) A radical hypothesis for neurodegeneration. Trends Neurosci 16:439–444
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45:117–127
Wang J-Y, Wen L-L, Huang Y-N et al (2006) Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr Pharm Des 12:3521–3533
Lu J, Zheng Y-L, Wu D-M et al (2007) Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem Pharmacol 74:1078–1090. https://doi.org/10.1016/j.bcp.2007.07.007
Mallidis C, Agbaje I, Rogers D et al (2007) Distribution of the receptor for advanced glycation end products in the human male reproductive tract: prevalence in men with diabetes mellitus. Hum Reprod 22:2169–2177. https://doi.org/10.1093/humrep/dem156
Chetsawang B, Govitrapong P, Ebadi M (2004) The neuroprotective effect of melatonin against the induction of c-Jun phosphorylation by 6-hydroxydopamine on SK-N-SH cells. Neurosci Lett 371:205–208. https://doi.org/10.1016/j.neulet.2004.08.068
Feng Z, Zhang J (2005) Long-term melatonin or 17beta-estradiol supplementation alleviates oxidative stress in ovariectomized adult rats. Free Radic Biol Med 39:195–204. https://doi.org/10.1016/j.freeradbiomed.2005.03.007
León J, Escames G, Rodríguez MI et al (2006) Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J Neurochem 98:2023–2033. https://doi.org/10.1111/j.1471-4159.2006.04029.x
Deng W-G, Tang S-T, Tseng H-P, Wu KK (2006) Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 108:518–524. https://doi.org/10.1182/blood-2005-09-3691
Wang X, Zheng W, Xie J-W et al (2010) Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegener 5:46. https://doi.org/10.1186/1750-1326-5-46
Chung S-Y, Han S-H (2003) Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J Pineal Res 34:95–102
Acknowledgments
The authors are grateful to the Pharmakon Neuroscience Research Network, Dhaka, Bangladesh.
Funding
The author(s) received no financial support for the research, authorship, and publication of this manuscript.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. MSU and GMA conceived the original idea and designed the outlines of the study. MFH, MSU, GMSU, and DMS wrote the draft of the manuscript. MSU prepared the figures of the manuscript. GEB, MSI, and BM reviewed the scientific contents of the manuscript. All authors read and approved the final submitted version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hossain, M.F., Uddin, M.S., Uddin, G.M.S. et al. Melatonin in Alzheimer’s Disease: A Latent Endogenous Regulator of Neurogenesis to Mitigate Alzheimer’s Neuropathology. Mol Neurobiol 56, 8255–8276 (2019). https://doi.org/10.1007/s12035-019-01660-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01660-3