Abstract
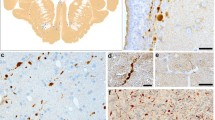
Axonal damage is a major factor contributing to disease progression in multiple sclerosis (MS) patients. On the histological level, acute axonal injury is most frequently analyzed by anti-amyloid precursor protein immunohistochemistry. To what extent this method truly detects axonal injury, and whether other proteins and organelles are as well subjected to axonal transport deficits in demyelinated tissues is not known. The aim of this study was to correlate ultrastructural morphology with the immunohistochemical appearance of acute axonal injury in a model of toxin-induced oligodendrocyte degeneration. C57BL/6J mice were intoxicated with 0.25% cuprizone to induce demyelination. The corpus callosum was investigated by serial block-face scanning electron microscopy (i.e., 3D EM), immunohistochemistry, and immunofluorescence microscopy. Brain tissues of progressive MS patients were included to test the relevance of our findings in mice for MS. Volumes of axonal swellings, determined by 3D EM, were comparable to volumes of axonal spheroids, determined by anti-APP immunofluorescence stains. Axonal swellings were present at myelinated and non-myelinated axonal internodes. Densities of amyloid precursor protein (APP)+ spheroids were highest during active demyelination. Besides APP, vesicular glutamate transporter 1 and mitochondrial proteins accumulated at sites of axonal spheroids. Such accumulations were found as well in lesions of progressive MS patients. In this correlative ultrastructural-immunohistochemical study, we provide strong evidence that breakdown of the axonal transport machinery results in focal accumulations of mitochondria and different synaptic proteins. We provide new marker proteins to visualize acute axonal injury, which helps to further understand the complex nature of axonal damage in progressive MS.








Similar content being viewed by others
References
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 46(4):907–911
Kipp M, Nyamoya S, Hochstrasser T, Amor S (2017) Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathol (Zurich, Switzerland) 27(2):123–137. https://doi.org/10.1111/bpa.12454
Hochstrasser T, Jiangshan Z, Ruhling S, Schmitz C, Kipp M (2018) Do pre-clinical multiple sclerosis models allow us to measure neurodegeneration and clinical progression? Expert Rev Neurother 18:1–3. https://doi.org/10.1080/14737175.2018.1459190
Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W (2002) Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain J Neurol 125(Pt 10):2202–2212
Schirmer L, Antel JP, Bruck W, Stadelmann C (2011) Axonal loss and neurofilament phosphorylation changes accompany lesion development and clinical progression in multiple sclerosis. Brain Pathol (Zurich, Switzerland) 21(4):428–440. https://doi.org/10.1111/j.1750-3639.2010.00466.x
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338(5):278–285. https://doi.org/10.1056/nejm199801293380502
Gudi V, Gai L, Herder V, Tejedor LS, Kipp M, Amor S, Suhs KW, Hansmann F et al (2017) Synaptophysin is a reliable marker for axonal damage. J Neuropathol Exp Neurol 76:109–125. https://doi.org/10.1093/jnen/nlw114
Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL et al (1990) Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A 87(4):1561–1565
Groemer TW, Thiel CS, Holt M, Riedel D, Hua Y, Huve J, Wilhelm BG, Klingauf J (2011) Amyloid precursor protein is trafficked and secreted via synaptic vesicles. PLoS One 6(4):e18754. https://doi.org/10.1371/journal.pone.0018754
Tyan SH, Shih AY, Walsh JJ, Maruyama H, Sarsoza F, Ku L, Eggert S, Hof PR et al (2012) Amyloid precursor protein (APP) regulates synaptic structure and function. Mol Cell Neurosci 51(1–2):43–52. https://doi.org/10.1016/j.mcn.2012.07.009
Sherriff FE, Bridges LR, Gentleman SM, Sivaloganathan S, Wilson S (1994) Markers of axonal injury in post mortem human brain. Acta Neuropathol 88(5):433–439
Stone JR, Singleton RH, Povlishock JT (2000) Antibodies to the C-terminus of the beta-amyloid precursor protein (APP): a site specific marker for the detection of traumatic axonal injury. Brain Res 871(2):288–302
Ferguson B, Matyszak MK, Esiri MM, Perry VH (1997) Axonal damage in acute multiple sclerosis lesions. Brain J Neurol 120(Pt 3):393–399
Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W (2000) Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain J Neurol 123(Pt 6):1174–1183
Hoflich KM, Beyer C, Clarner T, Schmitz C, Nyamoya S, Kipp M, Hochstrasser T (2016) Acute axonal damage in three different murine models of multiple sclerosis: a comparative approach. Brain Res 1650:125–133. https://doi.org/10.1016/j.brainres.2016.08.048
Buschmann JP, Berger K, Awad H, Clarner T, Beyer C, Kipp M (2012) Inflammatory response and chemokine expression in the white matter corpus callosum and gray matter cortex region during cuprizone-induced demyelination. Journal of molecular neuroscience : MN 48(1):66–76. https://doi.org/10.1007/s12031-012-9773-x
Kipp M, Clarner T, Dang J, Copray S, Beyer C (2009) The cuprizone animal model: new insights into an old story. Acta Neuropathol 118(6):723–736. https://doi.org/10.1007/s00401-009-0591-3
Vana AC, Flint NC, Harwood NE, Le TQ, Fruttiger M, Armstrong RC (2007) Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J Neuropathol Exp Neurol 66(11):975–988. https://doi.org/10.1097/NEN.0b013e3181587d46
Mason JL, Langaman C, Morell P, Suzuki K, Matsushima GK (2001) Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axonal calibre. Neuropathol Appl Neurobiol 27(1):50–58
Lindner M, Fokuhl J, Linsmeier F, Trebst C, Stangel M (2009) Chronic toxic demyelination in the central nervous system leads to axonal damage despite remyelination. Neurosci Lett 453(2):120–125. https://doi.org/10.1016/j.neulet.2009.02.004
Hennequin G, Agnes EJ, Vogels TP (2017) Inhibitory plasticity: balance, control, and codependence. Annu Rev Neurosci 40:557–579. https://doi.org/10.1146/annurev-neuro-072116-031005
Sheng M, Kim MJ (2002) Postsynaptic signaling and plasticity mechanisms. Science (New York, NY) 298(5594):776–780. https://doi.org/10.1126/science.1075333
Fremeau RT Jr, Voglmaier S, Seal RP, Edwards RH (2004) VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 27(2):98–103. https://doi.org/10.1016/j.tins.2003.11.005
McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM (1997) Identification and characterization of the vesicular GABA transporter. Nature 389(6653):870–876. https://doi.org/10.1038/39908
Liguz-Lecznar M, Skangiel-Kramska J (2007) Vesicular glutamate transporters (VGLUTs): the three musketeers of glutamatergic system. Acta Neurobiol Exp 67(3):207–218
Andrews H, White K, Thomson C, Edgar J, Bates D, Griffiths I, Turnbull D, Nichols P (2006) Increased axonal mitochondrial activity as an adaptation to myelin deficiency in the Shiverer mouse. J Neurosci Res 83(8):1533–1539. https://doi.org/10.1002/jnr.20842
Ohno N, Chiang H, Mahad DJ, Kidd GJ, Liu L, Ransohoff RM, Sheng ZH, Komuro H et al (2014) Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc Natl Acad Sci U S A 111(27):9953–9958. https://doi.org/10.1073/pnas.1401155111
Han SM, Baig HS, Hammarlund M (2016) Mitochondria localize to injured axons to support regeneration. Neuron 92(6):1308–1323. https://doi.org/10.1016/j.neuron.2016.11.025
Karbowski M, Neutzner A (2012) Neurodegeneration as a consequence of failed mitochondrial maintenance. Acta Neuropathol 123(2):157–171. https://doi.org/10.1007/s00401-011-0921-0
Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H (2001) GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33(1):72–86
Seewann A, Kooi EJ, Roosendaal SD, Barkhof F, van der Valk P, Geurts JJ (2009) Translating pathology in multiple sclerosis: the combination of postmortem imaging, histopathology and clinical findings. Acta Neurol Scand 119(6):349–355. https://doi.org/10.1111/j.1600-0404.2008.01137.x
Wagenknecht N, Becker B, Scheld M, Beyer C, Clarner T, Hochstrasser T, Kipp M (2016) Thalamus degeneration and inflammation in two distinct multiple sclerosis animal models. J Mol Neurosci : MN https://doi.org/10.1007/s12031-016-0790-z, 60, 102, 114
Hochstrasser T, Exner GL, Nyamoya S, Schmitz C, Kipp M (2017) Cuprizone-containing pellets are less potent to induce consistent demyelination in the corpus callosum of C57BL/6 mice. Journal of molecular neuroscience : MN 61(4):617–624. https://doi.org/10.1007/s12031-017-0903-3
Grosse-Veldmann R, Becker B, Amor S, van der Valk P, Beyer C, Kipp M (2015) Lesion expansion in experimental demyelination animal models and multiple sclerosis lesions. Mol Neurobiol 53:4905–4917. https://doi.org/10.1007/s12035-015-9420-y
Rock C, Zurita H, Lebby S, Wilson CJ, Apicella AJ (2018) Cortical circuits of callosal GABAergic neurons. Cerebral cortex (New York, NY : 1991) 28(4):1154–1167. https://doi.org/10.1093/cercor/bhx025
Brumovsky PR (2013) VGLUTs in Peripheral Neurons and the Spinal Cord: Time for a Review. ISRN Neurol. https://doi.org/10.1155/2013/829753
Ormel L, Stensrud MJ, Bergersen LH, Gundersen V (2012) VGLUT1 is localized in astrocytic processes in several brain regions. Glia 60:229–238. https://doi.org/10.1002/glia.21258
Bramlett HM, Kraydieh S, Green EJ, Dietrich WD (1997) Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J Neuropathol Exp Neurol 56(10):1132–1141
Herrero-Herranz E, Pardo LA, Gold R, Linker RA (2008) Pattern of axonal injury in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neurobiol Dis 30(2):162–173. https://doi.org/10.1016/j.nbd.2008.01.001
Sorbara CD, Wagner NE, Ladwig A, Nikic I, Merkler D, Kleele T, Marinkovic P, Naumann R et al (2014) Pervasive axonal transport deficits in multiple sclerosis models. Neuron 84(6):1183–1190. https://doi.org/10.1016/j.neuron.2014.11.006
Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D et al (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17(4):495–499. https://doi.org/10.1038/nm.2324
Lapato AS, Szu J, Hasselmann JP, Khalaj AJ, Binder DK, Tiwari-Woodruff SK (2017) Chronic demyelination-induced seizures. Neuroscience 346:409–422. https://doi.org/10.1016/j.neuroscience.2017.01.035
Rossi S, Muzio L, De Chiara V, Grasselli G, Musella A, Musumeci G, Mandolesi G, De Ceglia R et al (2011) Impaired striatal GABA transmission in experimental autoimmune encephalomyelitis. Brain Behav Immun 25(5):947–956. https://doi.org/10.1016/j.bbi.2010.10.004
Falco A, Pennucci R, Brambilla E, de Curtis I (2014) Reduction in parvalbumin-positive interneurons and inhibitory input in the cortex of mice with experimental autoimmune encephalomyelitis. Exp Brain Res 232(7):2439–2449. https://doi.org/10.1007/s00221-014-3944-7
Sheng ZH, Cai Q (2012) Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci 13(2):77–93. https://doi.org/10.1038/nrn3156
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. https://doi.org/10.1038/nature05292
Baltan S (2014) Excitotoxicity and mitochondrial dysfunction underlie age-dependent ischemic white matter injury. Advances in neurobiology 11:151–170. https://doi.org/10.1007/978-3-319-08894-5_8
Bando Y, Nomura T, Bochimoto H, Murakami K, Tanaka T, Watanabe T, Yoshida S (2015) Abnormal morphology of myelin and axon pathology in murine models of multiple sclerosis. Neurochem Int 81:16–27. https://doi.org/10.1016/j.neuint.2015.01.002
Rose J, Brian C, Woods J, Pappa A, Panayiotidis MI, Powers R, Franco R (2017) Mitochondrial dysfunction in glial cells: implications for neuronal homeostasis and survival. Toxicology 391:109–115. https://doi.org/10.1016/j.tox.2017.06.011
Acknowledgments
We thank Christiane Nolte and Helmut Kettenmann for providing hGFAP/EGFP transgenic mice (Max-Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany). We thank Sarah Wübbel, Astrid Baltruschat, and Beate Aschauer for their excellent and valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 643 kb)
Rights and permissions
About this article
Cite this article
Rühling, S., Kramer, F., Schmutz, S. et al. Visualization of the Breakdown of the Axonal Transport Machinery: a Comparative Ultrastructural and Immunohistochemical Approach. Mol Neurobiol 56, 3984–3998 (2019). https://doi.org/10.1007/s12035-018-1353-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1353-9