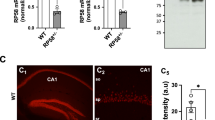
Abstract
In the Duchenne muscular dystrophy (DMD) syndrome, mutations affecting expression of Dp71, the main dystrophin isoform of the multipromoter dmd gene in brain, have been associated with intellectual disability and neuropsychiatric disturbances. Patients’ profile suggests alterations in prefrontal cortex-dependent executive processes, but the specific dysfunctions due to Dp71 deficiency are unclear. Dp71 is involved in brain ion homeostasis, and its deficiency is expected to increase neuronal excitability, which might compromise the integrity of neuronal networks undertaking high-order cognitive functions. Here, we used electrophysiological (patch clamp) and behavioral techniques in a transgenic mouse that display a selective loss of Dp71 and no muscular dystrophy, to identify changes in prefrontal cortex excitatory/inhibitory (E/I) balance and putative executive dysfunctions. We found prefrontal cortex E/I balance is shifted toward enhanced excitation in Dp71-null mice. This is associated with a selective alteration of AMPA receptor-mediated glutamatergic transmission and reduced synaptic plasticity, while inhibitory transmission is unaffected. Moreover, Dp71-null mice display deficits in cognitive processes that depend on prefrontal cortex integrity, such as cognitive flexibility and sensitivity of spatial working memory to proactive interference. Our data suggest that impaired cortical E/I balance and executive dysfunctions contribute to the intellectual and behavioral disturbances associated with Dp71 deficiency in DMD, in line with current neurobehavioral models considering these functions as key pathophysiological factors in various neurodevelopmental disorders. These new insights in DMD neurobiology also suggest new directions for therapeutic developments targeting excitatory neurotransmission, as well as for guidance of academic environment in severely affected DMD children.





Similar content being viewed by others
References
Lenk U, Hanke R, Thiele H, Speer A (1993) Point mutations at the carboxy terminus of the human dystrophin gene: implications for an association with mental retardation in DMD patients. Hum Mol Genet 2:1877–1881
Desguerre I, Christov C, Mayer M, Zeller R, Becane HM, Bastuji-Garin S, Leturcq F, Chiron C et al (2009) Clinical heterogeneity of Duchenne muscular dystrophy (DMD): definition of sub-phenotypes and predictive criteria by long-term follow-up. PLoS One 4:e4347
Taylor PJ, Betts GA, Maroulis S, Gilissen C, Pedersen RL, Mowat DR, Johnston HM, Buckley MF (2010) Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS One 5:e8803
Milic Rasic V, Vojinovic D, Pesovic J, Mijalkovic G, Lukic V, Mladenovic J, Kosac A, Novakovic I et al (2015) Intellectual ability in the Duchenne muscular dystrophy and dystrophin gene mutation location. Balkan J Med Genet 17:25–35
Moizard MP, Toutain A, Fournier D, Berret F, Raynaud M, Billard C, Andres C, Moraine C (2000) Severe cognitive impairment in DMD: obvious clinical indication for Dp71 isoform point mutation screening. Eur J Hum Genet 8:552–556
Daoud F, Angeard N, Demerre B, Martie I, Benyaou R, Leturcq F, Cossée M, Deburgrave N et al (2009a) Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Hum Mol Genet 18:3779–3794
De Brouwer AP, Nabuurs SB, Verhaart IE, Oudakker AR, Hordijk R, Yntema HG, Hordijk-Hos JM, Voesenek K et al (2014) A 3-base pair deletion, c.9711_9713del, in DMD results in intellectual disability without muscular dystrophy. Eur J Hum Genet 22:480–485
Snow WM, Anderson JE, Jakobson LS (2013) Neuropsychological and neurobehavioral functioning in Duchenne muscular dystrophy: A review. Neurosci Biobehav Rev 37:743–752
Mento G, Tarantino V, Bisiacchi PS (2011) The neuropsychological profile of infantile Duchenne muscular dystrophy. Clin Neuropsychol 25:1359–1377
Ricotti V, Mandy WP, Scoto M, Pane M, Deconinck N, Messina S, Mercuri E, Skuse DH et al (2016) Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev Med Child Neurol 58:77–84
Jones MW (2002) A comparative review of rodent prefrontal cortex and working memory. Curr Mol Med 2:639–647
Matzel LD, Kolata S (2010) Selective attention, working memory, and animal intelligence. Neurosci Biobehav Rev 34:23–30
Gatto CL, Broadie K (2010) Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci 2:4
Vinkers CH, Mirza NR, Olivier B, Kahn RS (2010) The inhibitory GABA system as a therapeutic target for cognitive symptoms in schizophrenia: investigational agents in the pipeline. Expert Opin Investig Drugs 19:1217–1233
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I et al (2011) Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477:171–178
Lederfein D, Levy Z, Augier N, Mornet D, Morris G, Fuchs O, Yaffe D, Nudel U (1992) A 71-kilodalton protein is a major product of the Duchenne muscular dystrophy gene in brain and other nonmuscle tissues. Proc Natl Acad Sci U S A 89:5346–5350
Waite A, Brown SC, Blake DJ (2012) The dystrophin-glycoprotein complex in brain development and disease. Trends Neurosci 35:487–496
Tadayoni R, Rendon A, Soria-Jasso LE, Cisneros B (2012) Dystrophin Dp71: the smallest but multifunctional product of the Duchenne muscular dystrophy gene. Mol Neurobiol 45:43–60
Hendriksen RG, Hoogland G, Schipper S, Hendriksen JG, Vles JS, Aalbers MW (2015) A possible role of dystrophin in neuronal excitability: a review of the current literature. Neurosci Biobehav Rev 51:255–262
Daoud F, Candelario-Martínez A, Billard JM, Avital A, Khelfaoui M, Rozenvald Y, Guegan M, Mornet D et al (2009b) Role of mental retardation-associated dystrophin-gene product Dp71 in excitatory synapse organization, synaptic plasticity and behavioral functions. PLoS One 4:e6574
Miranda R, Nudel U, Laroche S, Vaillend C (2011) Altered presynaptic ultrastructure in excitatory hippocampal synapses of mice lacking dystrophins Dp427 or Dp71. Neurobiol Dis 43:134–141
Sarig R, Mezger-Lallemand V, Gitelman I, Davis C, Fuchs O, Yaffe D, Nudel U (1999) Targeted inactivation of Dp71, the major non-muscle product of the DMD gene: differential activity of the Dp71 promoter during development. Hum Mol Genet 8:1–10
Chaussenot R, Edeline JM, Le Bec B, El Massioui N, Laroche S, Vaillend C (2015) Cognitive dysfunction in the dystrophin-deficient mouse model of Duchenne muscular dystrophy: a reappraisal from sensory to executive processes. Neurobiol Learn Mem 124:111–122
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Vaillend C, Perronnet C, Ros C, Gruszczynski C, Goyenvalle A, Laroche S, Danos O, Garcia L et al (2010) Rescue of a dystrophin-like protein by exon skipping in vivo restores GABAA-receptor clustering in the hippocampus of the mdx mouse. Mol Ther 18:1683–1688
Borg-Graham LJ, Monier C, Frégnac Y (1998) Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393:369–373
Monier C, Chavane F, Baudot P, Graham LJ, Frégnac Y (2003) Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron 37:663–680
Moreau AW, Amar M, Le Roux N, Morel N, Fossier P (2010) Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex 20:456–467
Meunier CN, Callebert J, Cancela JM, Fossier P (2015) Effect of dopaminergic D1 receptors on plasticity is dependent of serotoninergic 5-HT1A receptors in L5-pyramidal neurons of the prefrontal cortex. PLoS One 10:e0120286
Le Roux N, Amar M, Baux G, Fossier P (2006) Homeostatic control of the excitation-inhibition balance in cortical layer 5 pyramidal neurons. Eur J Neurosci 24:3507–3718
Haider B, Duque A, Hasenstaub AR, McCormick DA (2006) Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26:4535–4545
Higley MJ, Contreras D (2006) Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci 26:448–457
Abraham WC, Huggett A (1997) Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus 7:137–145
Touzani K, Puthanveettil SV, Kandel ER (2007) Consolidation of learning strategies during spatial working memory task requires protein synthesis in the prefrontal cortex. Proc Natl Acad Sci U S A 104:5632–5637
McCormick DA, Connors BW, Lighthall JW, Prince DA (1985) Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54:782–806
Connors BW, Gutnick MJ (1990) Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13:99–104
Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405
Katz B, Miledi R (1968) The role of calcium in neuromuscular facilitation. J Physiol 195:481–492
Connors NC, Kofuji P (2002) Dystrophin Dp71 is critical for the clustered localization of potassium channels in retinal glial cells. J Neurosci 22(11):4321–4327
Dalloz C, Sarig R, Fort P, Yaffe D, Bordais A, Pannicke T, Grosche J, Mornet D et al (2003) Targeted inactivation of dystrophin gene product Dp71: phenotypic impact in mouse retina. Hum Mol Genet 12(13):1543–1554
Fort PE, Sene A, Pannicke T, Roux MJ, Forster V, Mornet D, Nudel U, Yaffe D et al (2008) Kir4.1 and AQP4 associate with Dp71- and utrophin-DAPs complexes in specific and defined microdomains of Müller retinal glial cell membrane. Glia 56(6):597–610
Nicchia GP, Rossi A, Nudel U, Svelto M, Frigeri A (2008) Dystrophin-dependent and -independent AQP4 pools are expressed in the mouse brain. Glia 56(8):869–876
Sene A, Tadayoni R, Pannicke T, Wurm A, El Mathari B, Benard R, Roux MJ, Yaffe D et al (2009) Functional implication of Dp71 in osmoregulation and vascular permeability of the retina. PLoS One 4(10):e7329
Cia D, Simonutti M, Fort PE, Doly M, Rendon A (2014) Slight alteration of the electroretinogram in mice lacking dystrophin dp71. Ophthalmic Res 51(4):196–203
Vacca O, Darche M, Schaffer DV, Flannery JG, Sahel JA, Rendon A, Dalkara D (2014) AAV-mediated gene delivery in Dp71-null mouse model with compromised barriers. Glia 62(3):468–476
Vacca O, Charles-Messance H, El Mathari B, Sene A, Barbe P, Fouquet S, Aragón J, Darche M et al (2016) AAV-mediated gene therapy in dystrophin-Dp71 deficient mouse leads to blood-retinal barrier restoration and oedema reabsorption. Hum Mol Genet 25(14):3070–3079
De Bellis M, Pisani F, Mola MG, Rosito S, Simone L, Buccoliero C, Trojano M, Nicchia GP et al (2017) Translational readthrough generates new astrocyte AQP4 isoforms that modulate supramolecular clustering, glial endfeet localization, and water transport. Glia 65(5):790–803
Helleringer R, Le Verger D, Li X, Izabelle C, Chaussenot R, Belmaati-Cherkaoui M, Dammak R, Decottignies P, Daniel H, Galante M, Vaillend C (2018) Cerebellar synapse properties and cerebellum-dependent motor and non-motor performance in Dp71-null mice. Dis Model Mech 11(7).
Sicca F, Imbrici P, D'Adamo MC, Moro F, Bonatti F, Brovedani P, Grottesi A, Guerrini R et al (2011) Autism with seizures and intellectual disability: possible causative role of gain-of-function of the inwardly-rectifying K+ channel Kir4.1. Neurobiol Dis 43(1):239–247
Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL et al (2011) Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci 31(17):6392–6397
Scharfman HE, Binder DK (2013) Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochem Int 63(7):702–711
Sibille J, Pannasch U, Rouach N (2014) Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J Physiol 592(1):87–102
Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML (2016) The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 132(1):1–21
Hubbard JA, Szu JI, Binder DK (2018) The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull 136:118–129
Woo J, Kim JE, Im JJ, Lee J, Jeong HS, Park S, Jung SY, An H et al (2018) Astrocytic water channel aquaporin-4 modulates brain plasticity in both mice and humans: a potential gliogenetic mechanism underlying language-associated learning. Mol Psychiatry 23(4):1021–1030
Gorecki DC, Barnard EA (1995) Specific expression of G-dystrophin (Dp71) in the brain. Neuroreport 6:893–896
Aleman V, Osorio B, Chavez O, Rendon A, Mornet D, Martinez D (2001) Subcellular localization of Dp71 dystrophin isoforms in cultured hippocampal neurons and forebrain astrocytes. Histochem Cell Biol 115:243–254
Blake DJ, Hawkes R, Benson MA, Beesley PW (1999) Different dystrophin-like complexes are expressed in neurons and glia. J Cell Biol 147:645–658
Tozawa T, Itoh K, Yaoi T, Tando S, Umekage M, Dai H, Hosoi H, Fushiki S (2012) The shortest isoform of dystrophin (Dp40) interacts with a group of presynaptic proteins to form a presumptive novel complex in the mouse brain. Mol Neurobiol 45:287–297
Fujimoto T, Itoh K, Yaoi T, Fushiki S (2014) Somatodendritic and excitatory postsynaptic distribution of neuron-type dystrophin isoform, Dp40, in hippocampal neurons. Biochem Biophys Res Commun 452:79–84
Le Roux N, Amar M, Moreau A, Fossier P (2007) Involvement of NR2A- or NR2B-containing N-methyl-D-aspartate receptors in the potentiation of cortical layer 5 pyramidal neurone inputs depends on the developmental stage. Eur J Neurosci 26:289–301
Le Roux N, Amar M, Moreaux A, Fossier P (2008) Impaired GABAergic transmission disrupts normal homeostatic plasticity in rat cortical networks. Eur J Neurosci 27:3244–3256
Meunier C, Amar M, Lanfumey L, Hamon M, Fossier P (2013) 5-HT(1A) receptors direct the orientation of plasticity in layer 5 pyramidal neurons of the mouse prefrontal cortex. Neuropharm 71:37–45
Greger IH, Ziff EB, Penn AC (2007) Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci 30:407–416
Howe JR (2015) Modulation of non-NMDA receptor gating by auxiliary subunits. J Physiol 593:61–72
Losi G, Prybylowski K, Fu Z, Luo J, Wenthold RJ, Vicini S (2003) PSD-95 regulates NMDA receptors in developing cerebellar granule neurons of the rat. J Physiol 548:21–29
Thomson AM (2000) Facilitation, augmentation and potentiation at central synapses. Trends Neurosci 23:305–312
Fritschy JM (2008) Epilepsy, E/I balance and GABA(a) receptor plasticity. Front Mol Neurosci 1:5
Pozo K, Goda Y (2010) Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66:337–351
Kohl S, Heekeren K, Klosterkötter J, Kuhn J (2013) Prepulse inhibition in psychiatric disorders–apart from schizophrenia. J Psychiatr Res 47:445–452
Acknowledgements
We are grateful to the Zootechnic platform of our institute for mouse breeding, care, and genotyping and to Glenn Dallérac for the advice in electrophysiology.
Funding
This work was supported by Centre National de la Recherche Scientifique (CNRS, France) and University Paris-Sud (France), and by grants from Association Française contre les Myopathies (AFM, France; grant number 15299) and Agence Nationale de la Recherche (ANR, France; grant number ANR-14-CE13-0037-01) to C.V. and by a PhD fellowship from Ministère de l’Enseignement Supérieur et de la Recherche (France) to R.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experiments involving animals were undertaken following the guidelines of the European Directive 2010/63/EU, French National Committee (87/848), and local mouse facility (agreement #D91–471-104), with official approval (#2635) from the CEEA59 ethical committee (Comité d’éthique en matière d’expérimentation animale Paris Centre et Sud) and Ministère de l’Enseignement Supérieur et de la Recherche (France).
Conflict of Interest
The authors declare they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 215 kb)
Rights and permissions
About this article
Cite this article
Chaussenot, R., Amar, M., Fossier, P. et al. Dp71-Dystrophin Deficiency Alters Prefrontal Cortex Excitation-Inhibition Balance and Executive Functions. Mol Neurobiol 56, 2670–2684 (2019). https://doi.org/10.1007/s12035-018-1259-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1259-6