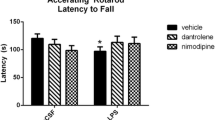
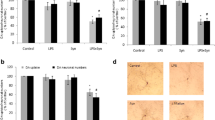
Abstract
Environmental toxicant exposure has been strongly implicated in the pathogenesis of Parkinson’s disease (PD). Clinical manifestations of non-motor and motor symptoms in PD stem from decades of progressive neurodegeneration selectively afflicting discrete neuronal populations along a caudo-rostral axis. However, recapitulating this spatiotemporal neurodegenerative pattern in rodents has been unsuccessful. The purpose of this study was to generate such animal PD models and delineate mechanism underlying the ascending neurodegeneration. Neuroinflammation, oxidative stress, and neuronal death in mice brains were measured at different times following a single systemic injection of lipopolysaccharide (LPS). We demonstrate that LPS produced an ascending neurodegeneration that temporally afflicted neurons initially in the locus coeruleus (LC), followed by substantia nigra, and lastly the primary motor cortex and hippocampus. To test the hypothesis that LPS-elicited early loss of noradrenergic LC neurons may underlie this ascending pattern, we used a neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) to deplete brain norepinephrine. DSP-4 injection resulted in a time-dependent ascending degenerative pattern similar to that generated by the LPS model. Mechanistic studies revealed that increase in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-2 (NOX2)-dependent superoxide/reactive oxygen species (ROS) production plays a key role in both LPS- and DSP-4-elicited neurotoxicity. We found that toxin-elicited chronic neuroinflammation, oxidative neuronal injuries, and neurodegeneration were greatly suppressed in mice deficient in NOX2 gene or treated with NOX2-specific inhibitor. Our studies document the first rodent PD model recapturing the ascending neurodegenerative pattern of PD patients and provide convincing evidence that the loss of brain norepinephrine is critical in initiating and maintaining chronic neuroinflammation and the discrete neurodegeneration in PD.








Similar content being viewed by others
Abbreviations
- 3-NT:
-
3-nitrotyrosine
- 4-HNE:
-
4-hydroxy-2-nonenal
- CPu:
-
caudate-putamen
- CD11b:
-
integrin αM chain
- DA:
-
dopamine
- DAMPs:
-
danger-associated molecular patterns
- DHE:
-
dihydroethidium
- DPI:
-
diphenyleneiodonium
- DSP-4:
-
N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine
- FDG:
-
fludeoxyglucose
- Hip:
-
hippocampus
- Iba-1:
-
ionized calcium-binding adapter molecule-1
- LC:
-
locus coeruleus
- LPS:
-
lipopolysaccharide
- MCtx:
-
motor cortex
- NE:
-
norepinephrine
- NeuN:
-
neuronal nuclei
- NOX2:
-
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-2
- PBR:
-
peripheral benzodiazepine receptor
- PET:
-
positron emission tomography
- ROS:
-
reactive oxygen species
- SNpc :
-
substantia nigra pars compacta
- SUV:
-
standard uptake value
- TH:
-
tyrosine hydroxylase
- TSPO:
-
translocator protein
- VTA:
-
ventral tegmental area
References
Cummings JL (1992) Depression and Parkinson's disease: a review. Am J Psychiatry 149(4):443–454. https://doi.org/10.1176/ajp.149.4.443
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128(Pt 6):1314–1322. https://doi.org/10.1093/brain/awh445
Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Knopman D, Graff-Radford N et al (2010) Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 133(Pt 2):540–556. https://doi.org/10.1093/brain/awp280
Hanganu A, Bedetti C, Degroot C, Mejia-Constain B, Lafontaine AL, Soland V, Chouinard S, Bruneau MA et al (2014) Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson's disease longitudinally. Brain 137(Pt 4):1120–1129. https://doi.org/10.1093/brain/awu036
Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G et al (2014) Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain 137(Pt 9):2493–2508. https://doi.org/10.1093/brain/awu193
Halliday GM, Leverenz JB, Schneider JS, Adler CH (2014) The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord 29(5):634–650
Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, Hasegawa T, Sugeno N et al (2012) Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson's disease: a 3 year longitudinal study. Brain 135(Pt 1):161–169. https://doi.org/10.1093/brain/awr321
Arai E, Arai M, Uchiyama T, Higuchi Y, Aoyagi K, Yamanaka Y, Yamamoto T, Nagano O et al (2012) Subthalamic deep brain stimulation can improve gastric emptying in Parkinson's disease. Brain 135(Pt 5):1478–1485. https://doi.org/10.1093/brain/aws086
Jellinger KA (1991) Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14(3):153–197
Rinne JO, Rummukainen J, Paljarvi L, Rinne UK (1989) Dementia in Parkinson's disease is related to neuronal loss in the medial substantia nigra. Ann Neurol 26(1):47–50. https://doi.org/10.1002/ana.410260107
Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J (1996) Pattern of brain destruction in Parkinson's and Alzheimer's diseases. J Neural Transm (Vienna) 103(4):455–490. https://doi.org/10.1007/BF01276421
Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60(3):337–341
Buchman AS, Nag S, Shulman JM, Lim AS, VanderHorst VG, Leurgans SE, Schneider JA, Bennett DA (2012) Locus coeruleus neuron density and parkinsonism in older adults without Parkinson's disease. Mov Disord 27(13):1625–1631. https://doi.org/10.1002/mds.25142
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24(2):197–211
Gispert S, Del Turco D, Garrett L, Chen A, Bernard DJ, Hamm-Clement J, Korf HW, Deller T et al (2003) Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol Cell Neurosci 24(2):419–429
Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK (2006) Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci 26(1):41–50. https://doi.org/10.1523/JNEUROSCI.4308-05.2006
Sacino AN, Brooks M, Thomas MA, McKinney AB, McGarvey NH, Rutherford NJ, Ceballos-Diaz C, Robertson J et al (2014) Amyloidogenic alpha-synuclein seeds do not invariably induce rapid, widespread pathology in mice. Acta Neuropathol 127(5):645–665. https://doi.org/10.1007/s00401-014-1268-0
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55(5):453–462. https://doi.org/10.1002/glia.20467
Qin LY, Liu YX, Hong JS, Crews FT (2013) NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia 61(6):855–868. https://doi.org/10.1002/glia.22479
Wang Q, Qian L, Chen SH, Chu CH, Wilson B, Oyarzabal E, Ali S, Robinson B et al (2015) Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson's disease models. Brain 138(Pt 5):1247–1262. https://doi.org/10.1093/brain/awv034
Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL (2004) Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflammation 1(1):9. https://doi.org/10.1186/1742-2094-1-9
Biber K, Neumann H, Inoue K, Boddeke HW (2007) Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci 30(11):596–602. https://doi.org/10.1016/j.tins.2007.08.007
Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D (2007) Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A 104(34):13804–13809. https://doi.org/10.1073/pnas.0702753104
Jiang L, Chen SH, Chu CH, Wang SJ, Oyarzabal E, Wilson B, Sanders V, Xie K et al (2015) A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia 63(6):1057–1072. https://doi.org/10.1002/glia.22801
Cai Z, Chattopadhyay N, Liu WJ, Chan C, Pignol JP, Reilly RM (2011) Optimized digital counting colonies of clonogenic assays using ImageJ software and customized macros: comparison with manual counting. Int J Radiat Biol 87(11):1135–1146. https://doi.org/10.3109/09553002.2011.622033
Brooks DJ (2008) Technology insight: imaging neurodegeneration in Parkinson's disease. Nat Clin Pract Neurol 4(5):267–277. https://doi.org/10.1038/ncpneuro0773
Mosconi L (2013) Glucose metabolism in normal aging and Alzheimer's disease: methodological and physiological considerations for PET studies. Clin Transl Imaging 1(4):217–233. https://doi.org/10.1007/s40336-013-0026-y
Barber TR, Klein JC, Mackay CE, Hu MTM (2017) Neuroimaging in pre-motor Parkinson's disease. Neuroimage Clin 15:215–227. https://doi.org/10.1016/j.nicl.2017.04.011
Mirrione MM, Schiffer WK, Fowler JS, Alexoff DL, Dewey SL, Tsirka SE (2007) A novel approach for imaging brain-behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. Neuroimage 38(1):34–42. https://doi.org/10.1016/j.neuroimage.2007.06.032
Gassmann M, Grenacher B, Rohde B, Vogel J (2009) Quantifying Western blots: pitfalls of densitometry. Electrophoresis 30(11):1845–1855. https://doi.org/10.1002/elps.200800720
Goto S, Hirano A, Matsumoto S (1990) Immunohistochemical study of the striatal efferents and nigral dopaminergic neurons in parkinsonism-dementia complex on Guam in comparison with those in Parkinson's and Alzheimer's diseases. Ann Neurol 27(5):520–527. https://doi.org/10.1002/ana.410270511
Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ (2005) Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 64(3):545–547. https://doi.org/10.1212/01.WNL.0000150591.33787.A4
Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20(16):6309–6316
Szot P, Miguelez C, White SS, Franklin A, Sikkema C, Wilkinson CW, Ugedo L, Raskind MA (2010) A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat. Neuroscience 166(1):279–291. https://doi.org/10.1016/j.neuroscience.2009.12.027
Ignatowski TA, Noble BK, Wright JR, Gorfien JL, Heffner RR, Spengler RN (1997) Neuronal-associated tumor necrosis factor (TNF alpha): its role in noradrenergic functioning and modification of its expression following antidepressant drug administration. J Neuroimmunol 79(1):84–90
Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O'Banion MK, Weinberg G, Klockgether T et al (2002) Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer's disease. J Neurosci 22(7):2434–2442
Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, Walker Z, Turkheimer FE et al (2013) Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacol 38(6):938–949. https://doi.org/10.1038/npp.2012.255
Brown GC, Neher JJ (2014) Microglial phagocytosis of live neurons. Nat Rev Neurosci 15(4):209–216. https://doi.org/10.1038/nrn3710
Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS (2011) Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson's disease. Environ Health Perspect 119(6):807–814. https://doi.org/10.1289/ehp.1003013
Chen SH, Oyarzabal EA, Hong JS (2016) Critical role of the Mac1/NOX2 pathway in mediating reactive microgliosis-generated chronic neuroinflammation and progressive neurodegeneration. Curr Opin Pharmacol 26:54–60. https://doi.org/10.1016/j.coph.2015.10.001
Elstner M, Muller SK, Leidolt L, Laub C, Krieg L, Schlaudraff F, Liss B, Morris C et al (2011) Neuromelanin, neurotransmitter status and brainstem location determine the differential vulnerability of catecholaminergic neurons to mitochondrial DNA deletions. Mol Brain 4:43. https://doi.org/10.1186/1756-6606-4-43
Sanchez-Padilla J, Guzman JN, Ilijic E, Kondapalli J, Galtieri DJ, Yang B, Schieber S, Oertel W et al (2014) Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat Neurosci 17(6):832–840. https://doi.org/10.1038/nn.3717
Tong J, Hornykiewicz O, Kish SJ (2006) Inverse relationship between brain noradrenaline level and dopamine loss in Parkinson disease: a possible neuroprotective role for noradrenaline. Arch Neurol 63(12):1724–1728. https://doi.org/10.1001/archneur.63.12.1724
Borodovitsyna O, Flamini M, Chandler D (2017) Noradrenergic modulation of cognition in health and disease. Neural Plast 2017:6031478:1–14. https://doi.org/10.1155/2017/6031478
Lookingland KJ, Chapin DS, McKay DW, Moore KE (1986) Comparative effects of the neurotoxins N-chloroethyl-N-ethyl-2-bromobenzylamine hydrochloride (DSP4) and 6-hydroxydopamine on hypothalamic noradrenergic, dopaminergic and 5-hydroxytryptaminergic neurons in the male rat. Brain Res 365(2):228–234
Fornai F, Alessandri MG, Torracca MT, Bassi L, Corsini GU (1997) Effects of noradrenergic lesions on MPTP/MPP+ kinetics and MPTP-induced nigrostriatal dopamine depletions. J Pharmacol Exp Ther 283(1):100–107
Perez V, Sosti V, Rubio A, Barbanoj M, Gich I, Rodriguez-Alvarez J, Kulisevsky J (2009) Noradrenergic modulation of the motor response induced by long-term levodopa administration in parkinsonian rats. J Neural Transm (Vienna) 116(7):867–874. https://doi.org/10.1007/s00702-009-0242-9
Ostock CY, Lindenbach D, Goldenberg AA, Kampton E, Bishop C (2014) Effects of noradrenergic denervation by anti-DBH-saporin on behavioral responsivity to L-DOPA in the hemi-parkinsonian rat. Behav Brain Res 270:75–85. https://doi.org/10.1016/j.bbr.2014.05.009
Qian L, Wu HM, Chen SH, Zhang D, Ali SF, Peterson L, Wilson B, Lu RB et al (2011) Beta 2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway. J Immunol 186(7):4443–4454. https://doi.org/10.4049/jimmunol.1002449
Mittal S, Bjornevik K, Im DS, Flierl A, Dong X, Locascio JJ, Abo KM, Long E et al (2017) Beta2-adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson's disease. Science 357(6354):891–898. https://doi.org/10.1126/science.aaf3934
Madrigal JL, Feinstein DL, Dello Russo C (2005) Norepinephrine protects cortical neurons against microglial-induced cell death. J Neurosci Res 81(3):390–396. https://doi.org/10.1002/jnr.20481
Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J et al (2010) Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A 107(13):6058–6063. https://doi.org/10.1073/pnas.0909586107
Gyoneva S, Traynelis SF (2013) Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem 288(21):15291–15302. https://doi.org/10.1074/jbc.M113.458901
Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, Jacobs AH, Herholz K et al (2005) Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 65(11):1716–1722. https://doi.org/10.1212/01.wnl.0000191154.78131.f6
Braak H, Rub U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110(5):517–536. https://doi.org/10.1007/s00702-002-0808-2
Dickson DW, Schmidt ML, Lee VM, Zhao ML, Yen SH, Trojanowski JQ (1994) Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol 87(3):269–276
Pereira JB, Junque C, Bartres-Faz D, Ramirez-Ruiz B, Marti MJ, Tolosa E (2013) Regional vulnerability of hippocampal subfields and memory deficits in Parkinson's disease. Hippocampus 23(8):720–728. https://doi.org/10.1002/hipo.22131
Parent M, Parent A (2006) Relationship between axonal collateralization and neuronal degeneration in basal ganglia. J Neural Transm Suppl 70:85–88
Song S, Chen SH, Moy S, Wang QS, Hong JS (2017) Norepinephrine deficiency accelerates ascending sequential neurodegeneration and progression of non-motor/motor symptoms in an inflammatory Parkinson’s diseases mouse model. In: Society for neuroscience annual meeting, Washington D.C.
Sanders LH, Greenamyre JT (2013) Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic Biol Med 62:111–120. https://doi.org/10.1016/j.freeradbiomed.2013.01.003
Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2(12). https://doi.org/10.3389/fnagi.2010.00012
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson's disease. Free Radic Biol Med 62:13–25. https://doi.org/10.1016/j.freeradbiomed.2013.05.001
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18(2):101–113. https://doi.org/10.1038/nrn.2016.178
Surmeier DJ, Schumacker PT (2013) Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease. J Biol Chem 288(15):10736–10741. https://doi.org/10.1074/jbc.R112.410530
Yang TT, Lin C, Hsu CT, Wang TF, Ke FY, Kuo YM (2013) Differential distribution and activation of microglia in the brain of male C57BL/6J mice. Brain Struct Funct 218(4):1051–1060. https://doi.org/10.1007/s00429-012-0446-x
Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468(7324):696–U119. https://doi.org/10.1038/nature09536
Surmeier DJ, Guzman JN, Sanchez-Padilla J, Schumacker PT (2011) The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson's disease. Neuroscience 198:221–231. https://doi.org/10.1016/j.neuroscience.2011.08.045
Goldberg JA, Guzman JN, Estep CM, Ilijic E, Kondapalli J, Sanchez-Padilla J, Surmeier DJ (2012) Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson's disease. Nat Neurosci 15(10):1414–1421. https://doi.org/10.1038/nn.3209
Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J et al (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science 357(6357):1255–1261. https://doi.org/10.1126/science.aam9080
Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease—a double-edged sword. Neuron 35(3):419–432
Gilgun-Sherki Y, Melamed E, Offen D (2006) Anti-inflammatory drugs in the treatment of neurodegenerative diseases: current state. Curr Pharm Des 12(27):3509–3519
Liu B, Jiang JW, Wilson BC, Du L, Yang SN, Wang JY, Wu GC, Cao XD et al (2000) Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J Pharmacol Exp Ther 295(1):125–132
Acknowledgments
This research was supported through the Intramural Research Program at the National Institute of Environmental Health Sciences in the National Institutes of Health, USA. We thank Anthony Lockhart for assistance with animal colony management and maintenance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
The authors declare that they have no actual or potential competing financial interests.
Electronic supplementary material
ESM 1
(PDF 675 kb)
Rights and permissions
About this article
Cite this article
Song, S., Jiang, L., Oyarzabal, E.A. et al. Loss of Brain Norepinephrine Elicits Neuroinflammation-Mediated Oxidative Injury and Selective Caudo-Rostral Neurodegeneration. Mol Neurobiol 56, 2653–2669 (2019). https://doi.org/10.1007/s12035-018-1235-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1235-1