Abstract
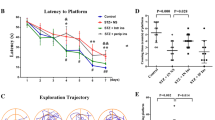
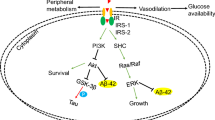
Impairment of biliverdin reductase-A (BVR-A) is an early event leading to brain insulin resistance in AD. Intranasal insulin (INI) administration is under evaluation as a strategy to alleviate brain insulin resistance; however, the molecular mechanisms underlying INI beneficial effects are still unclear. We show that INI improves insulin signaling activation in the hippocampus and cortex of adult and aged 3×Tg-AD mice by ameliorating BVR-A activation. These changes were associated with a reduction of nitrosative stress, Tau phosphorylation, and Aβ oligomers in brain, along with improved cognitive functions. The role of BVR-A was strengthened by showing that cells lacking BVR-A: (i) develop insulin resistance if treated with insulin and (ii) can be recovered from insulin resistance only if treated with a BVR-A-mimetic peptide. These novel findings shed light on the mechanisms underlying INI treatment effects and suggest BVR-A as potential therapeutic target to prevent brain insulin resistance in AD.












Similar content being viewed by others
References
De Felice FG (2013) Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest 123(2):531–539. https://doi.org/10.1172/JCI64595
Biessels GJ, Reagan LP (2015) Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 16(11):660–671. https://doi.org/10.1038/nrn4019
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA (2015) Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133(5):739–749. https://doi.org/10.1111/jnc.13037
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7(2):85–96. https://doi.org/10.1038/nrm1837
Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD (2005) Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc Natl Acad Sci U S A 102(20):7109–7114. https://doi.org/10.1073/pnas.0502173102
Gibbs PE, Lerner-Marmarosh N, Poulin A, Farah E, Maines MD (2014) Human biliverdin reductase-based peptides activate and inhibit glucose uptake through direct interaction with the kinase domain of insulin receptor. FASEB J 28(6):2478–2491. https://doi.org/10.1096/fj.13-247015
Lerner-Marmarosh N, Miralem T, Gibbs PE, Maines MD (2008) Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc Natl Acad Sci U S A 105(19):6870–6875. https://doi.org/10.1073/pnas.0800750105
Miralem T, Lerner-Marmarosh N, Gibbs PE, Jenkins JL, Heimiller C, Maines MD (2016) Interaction of human biliverdin reductase with Akt/protein kinase B and phosphatidylinositol-dependent kinase 1 regulates glycogen synthase kinase 3 activity: a novel mechanism of Akt activation. FASEB J 30(8):2926–2944. https://doi.org/10.1096/fj.201600330RR
Zhang Y, Zong W, Zhang L, Ma Y, Wang J (2016) Protein kinase M zeta and the maintenance of long-term memory. Neurochem Int 99:215–220. https://doi.org/10.1016/j.neuint.2016.07.007
Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, Perluigi M, Mancuso C et al (2011) Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer's disease and amnestic mild cognitive impairment. J Alzheimers Dis 25(4):623–633. https://doi.org/10.3233/JAD-2011-110092
Barone E, Di Domenico F, Cenini G, Sultana R, Cini C, Preziosi P, Perluigi M, Mancuso C et al (2011) Biliverdin reductase—a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim Biophys Acta 1812(4):480–487. https://doi.org/10.1016/j.bbadis.2011.01.005
Barone E, Di Domenico F, Cassano T, Arena A, Tramutola A, Lavecchia MA, Coccia R, Butterfield DA et al (2016) Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: a new paradigm. Free Radic Biol Med 91:127–142. https://doi.org/10.1016/j.freeradbiomed.2015.12.012
Hanson LR, Frey WH 2nd (2007) Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J NeuroImmune Pharmacol 2(1):81–86. https://doi.org/10.1007/s11481-006-9039-x
Reger MA, Watson GS, Frey WH 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA et al (2006) Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27(3):451–458. https://doi.org/10.1016/j.neurobiolaging.2005.03.016
Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR et al (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70(6):440–448. https://doi.org/10.1212/01.WNL.0000265401.62434.36
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M et al (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69(1):29–38. https://doi.org/10.1001/archneurol.2011.233
Apostolatos A, Song S, Acosta S, Peart M, Watson JE, Bickford P, Cooper DR, Patel NA (2012) Insulin promotes neuronal survival via the alternatively spliced protein kinase CdeltaII isoform. J Biol Chem 287(12):9299–9310. https://doi.org/10.1074/jbc.M111.313080
Mao YF, Guo Z, Zheng T, Jiang Y, Yan Y, Yin X, Chen Y, Zhang B (2016) Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell 15(5):893–902. https://doi.org/10.1111/acel.12498
Salameh TS, Bullock KM, Hujoel IA, Niehoff ML, Wolden-Hanson T, Kim J, Morley JE, Farr SA et al (2015) Central nervous system delivery of intranasal insulin: mechanisms of uptake and effects on cognition. J Alzheimers Dis 47(3):715–728. https://doi.org/10.3233/JAD-150307
Zhang Y, Dai CL, Chen Y, Iqbal K, Liu F, Gong CX (2016) Intranasal insulin prevents anesthesia-induced spatial learning and memory deficit in mice. Sci Rep 6:21186. https://doi.org/10.1038/srep21186
Chen Y, Dai CL, Wu Z, Iqbal K, Liu F, Zhang B, Gong CX (2017) Intranasal insulin prevents anesthesia-induced cognitive impairment and chronic neurobehavioral changes. Front Aging Neurosci 9:136. https://doi.org/10.3389/fnagi.2017.00136
Nedelcovych MT, Gadiano AJ, Wu Y, Manning AA, Thomas AG, Khuder SS, Yoo SW, Xu J et al (2018) Pharmacokinetics of intranasal versus subcutaneous insulin in the mouse. ACS Chem Neurosci 9:809–816. https://doi.org/10.1021/acschemneuro.7b00434
Lochhead JJ, Thorne RG (2012) Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 64(7):614–628. https://doi.org/10.1016/j.addr.2011.11.002
Chauhan MB, Chauhan NB (2015) Brain uptake of neurotherapeutics after intranasal versus intraperitoneal delivery in mice. J Neurol Neurosurg 2 (1)
Stanley M, Macauley SL, Holtzman DM (2016) Changes in insulin and insulin signaling in Alzheimer’s disease: cause or consequence? J Exp Med 213(8):1375–1385. https://doi.org/10.1084/jem.20160493
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP et al (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421
Cassano T, Romano A, Macheda T, Colangeli R, Cimmino CS, Petrella A, LaFerla FM, Cuomo V et al (2011) Olfactory memory is impaired in a triple transgenic model of Alzheimer disease. Behav Brain Res 224(2):408–412. https://doi.org/10.1016/j.bbr.2011.06.029
Cassano T, Serviddio G, Gaetani S, Romano A, Dipasquale P, Cianci S, Bellanti F, Laconca L et al (2012) Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol Aging 33(6):1121 e1121–1112. https://doi.org/10.1016/j.neurobiolaging.2011.09.021
Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, Gobbi G (2010) Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 35(10):2083–2100. https://doi.org/10.1038/npp.2010.80
Romano A, Pace L, Tempesta B, Lavecchia AM, Macheda T, Bedse G, Petrella A, Cifani C et al (2014) Depressive-like behavior is paired to monoaminergic alteration in a murine model of Alzheimer’s disease. Int J Neuropsychopharmacol 18(4):pyu020. https://doi.org/10.1093/ijnp/pyu020
Scuderi C, Bronzuoli MR, Facchinetti R, Pace L, Ferraro L, Broad KD, Serviddio G, Bellanti F et al (2018) Ultramicronized palmitoylethanolamide rescues learning and memory impairments in a triple transgenic mouse model of Alzheimer’s disease by exerting anti-inflammatory and neuroprotective effects. Transl Psychiatry 8(1):32. https://doi.org/10.1038/s41398-017-0076-4
Giustino A, Cagiano R, Carratu MR, Cassano T, Tattoli M, Cuomo V (1999) Prenatal exposure to low concentrations of carbon monoxide alters habituation and non-spatial working memory in rat offspring. Brain Res 844(1–2):201–205
Ripoli C, Piacentini R, Riccardi E, Leone L, Li Puma DD, Bitan G, Grassi C (2013) Effects of different amyloid beta-protein analogues on synaptic function. Neurobiol Aging 34(4):1032–1044. https://doi.org/10.1016/j.neurobiolaging.2012.06.027
Ripoli C, Cocco S, Li Puma DD, Piacentini R, Mastrodonato A, Scala F, Puzzo D, D'Ascenzo M et al (2014) Intracellular accumulation of amyloid-beta (Abeta) protein plays a major role in Abeta-induced alterations of glutamatergic synaptic transmission and plasticity. J Neurosci 34(38):12893–12903. https://doi.org/10.1523/JNEUROSCI.1201-14.2014
Mayer CM, Belsham DD (2010) Central insulin signaling is attenuated by long-term insulin exposure via insulin receptor substrate-1 serine phosphorylation, proteasomal degradation, and lysosomal insulin receptor degradation. Endocrinology 151(1):75–84. https://doi.org/10.1210/en.2009-0838
Miralem T, Lerner-Marmarosh N, Gibbs PE, Tudor C, Hagen FK, Maines MD (2012) The human biliverdin reductase-based peptide fragments and biliverdin regulate protein kinase Cdelta activity: the peptides are inhibitors or substrate for the protein kinase C. J Biol Chem 287(29):24698–24712. https://doi.org/10.1074/jbc.M111.326504
Cenini G, Sultana R, Memo M, Butterfield DA (2008) Effects of oxidative and nitrosative stress in brain on p53 proapoptotic protein in amnestic mild cognitive impairment and Alzheimer disease. Free Radic Biol Med 45(1):81–85. https://doi.org/10.1016/j.freeradbiomed.2008.03.015
Salim M, Brown-Kipphut BA, Maines MD (2001) Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J Biol Chem 276(14):10929–10934. https://doi.org/10.1074/jbc.M010753200
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR et al (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122(4):1316–1338. https://doi.org/10.1172/JCI59903
Copps KD, White MF (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55(10):2565–2582. https://doi.org/10.1007/s00125-012-2644-8
Kapitulnik J, Maines MD (2009) Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci 30(3):129–137. https://doi.org/10.1016/j.tips.2008.12.003
Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S et al (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14:168–181. https://doi.org/10.1038/nrneurol.2017.185
King GL, Park K, Li Q (2016) Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award Lecture. Diabetes 65(6):1462–1471. https://doi.org/10.2337/db16-0152
Ge X, Yu Q, Qi W, Shi X, Zhai Q (2008) Chronic insulin treatment causes insulin resistance in 3T3-L1 adipocytes through oxidative stress. Free Radic Res 42(6):582–591. https://doi.org/10.1080/10715760802158448
Bell GA, Fadool DA (2017) Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice. Physiol Behav 174:104–113. https://doi.org/10.1016/j.physbeh.2017.02.044
Adzovic L, Lynn AE, D'Angelo HM, Crockett AM, Kaercher RM, Royer SE, Hopp SC, Wenk GL (2015) Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation 12:63. https://doi.org/10.1186/s12974-015-0282-z
Lerner-Marmarosh N, Miralem T, Gibbs PE, Maines MD (2007) Regulation of TNF-alpha-activated PKC-zeta signaling by the human biliverdin reductase: identification of activating and inhibitory domains of the reductase. FASEB J 21(14):3949–3962. https://doi.org/10.1096/fj.07-8544com
Tundo GR, Sbardella D, Ciaccio C, Grasso G, Gioia M, Coletta A, Polticelli F, Di Pierro D et al (2017) Multiple functions of insulin-degrading enzyme: a metabolic crosslight? Crit Rev Biochem Mol Biol 52(5):554–582. https://doi.org/10.1080/10409238.2017.1337707
Association As (2017) Alzheimer’s disease facts and figures. Alzheimers Dement 13:325–373
Diehl T, Mullins R, Kapogiannis D (2017) Insulin resistance in Alzheimer’s disease. Transl Res 183:26–40. https://doi.org/10.1016/j.trsl.2016.12.005
Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ (2010) Sex differences in beta-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res 1366:233–245. https://doi.org/10.1016/j.brainres.2010.10.009
Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F et al (2008) Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res 1216:92–103. https://doi.org/10.1016/j.brainres.2008.03.079
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24(8):1063–1070
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H et al (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated Abeta oligomers. J Clin Invest 122(4):1339–1353. https://doi.org/10.1172/JCI57256
Di Domenico F, Barone E, Perluigi M, Butterfield DA (2017) The triangle of death in Alzheimer’s disease brain: the aberrant cross-talk among energy metabolism, mammalian target of rapamycin signaling, and protein homeostasis revealed by redox proteomics. Antioxid Redox Signal 26(8):364–387. https://doi.org/10.1089/ars.2016.6759
Barone E, Di Domenico F, Mancuso C, Butterfield DA (2014) The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: it’s time for reconciliation. Neurobiol Dis 62:144–159. https://doi.org/10.1016/j.nbd.2013.09.018
Tramutola A, Lanzillotta C, Arena A, Barone E, Perluigi M, Di Domenico F (2016) Increased mammalian target of rapamycin signaling contributes to the accumulation of protein oxidative damage in a mouse model of Down’s syndrome. Neurodegener Dis 16(1–2):62–68. https://doi.org/10.1159/000441419
Di Domenico F, Barone E, Mancuso C, Perluigi M, Cocciolo A, Mecocci P, Butterfield DA, Coccia R (2012) HO-1/BVR-a system analysis in plasma from probable Alzheimer's disease and mild cognitive impairment subjects: a potential biochemical marker for the prediction of the disease. J Alzheimers Dis 32(2):277–289. https://doi.org/10.3233/JAD-2012-121045
Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC (1987) Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science 235(4791):873–877
Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP (1991) Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res 10(4):299–305
Triani F, Tramutola A, Di Domenico F, Sharma N, Butterfield DA, Head E, Perluigi M, Barone E (2018) Biliverdin reductase-A impairment links brain insulin resistance with increased Aβ production in an animal model of aging: implications for Alzheimer disease. Biochim Biophys Acta (BBA) Mol Basis Dis doi:https://doi.org/10.1016/j.bbadis.2018.07.005
Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM et al (2008) Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis 13(3):323–331
Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W (2007) Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32(1):239–243. https://doi.org/10.1038/sj.npp.1301193
Chapman CD, Frey WH 2nd, Craft S, Danielyan L, Hallschmid M, Schioth HB, Benedict C (2013) Intranasal treatment of central nervous system dysfunction in humans. Pharm Res 30(10):2475–2484. https://doi.org/10.1007/s11095-012-0915-1
Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE et al (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25(34):7709–7717. https://doi.org/10.1523/JNEUROSCI.2177-05.2005
Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME et al (2010) Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta ) deposition. Proc Natl Acad Sci U S A 107(41):17763–17767. https://doi.org/10.1073/pnas.1010461107
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM (2011) Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 14(6):750–756. https://doi.org/10.1038/nn.2801
Oh H, Madison C, Baker S, Rabinovici G, Jagust W (2016) Dynamic relationships between age, amyloid-beta deposition, and glucose metabolism link to the regional vulnerability to Alzheimer’s disease. Brain 139(Pt 8):2275–2289. https://doi.org/10.1093/brain/aww108
Willette AA, Modanlo N, Kapogiannis D, Alzheimer's Disease Neuroimaging I (2015) Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes 64(6):1933–1940. https://doi.org/10.2337/db14-1507
Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA (2009) Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci 29(20):6734–6751. https://doi.org/10.1523/JNEUROSCI.1350-09.2009
van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM (2005) Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem 94(4):1158–1166. https://doi.org/10.1111/j.1471-4159.2005.03269.x
Chiu SL, Chen CM, Cline HT (2008) Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58(5):708–719. https://doi.org/10.1016/j.neuron.2008.04.014
Lee CC, Huang CC, Hsu KS (2011) Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology 61(4):867–879. https://doi.org/10.1016/j.neuropharm.2011.06.003
Bedse G, Di Domenico F, Serviddio G, Cassano T (2015) Aberrant insulin signaling in Alzheimer's disease: current knowledge. Front Neurosci 9:204. https://doi.org/10.3389/fnins.2015.00204
Carlson CJ, White MF, Rondinone CM (2004) Mammalian target of rapamycin regulates IRS-1 serine 307 phosphorylation. Biochem Biophys Res Commun 316(2):533–539. https://doi.org/10.1016/j.bbrc.2004.02.082
Perluigi M, Pupo G, Tramutola A, Cini C, Coccia R, Barone E, Head E, Butterfield DA et al (2014) Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome brain. Biochim Biophys Acta 1842(7):1144–1153. https://doi.org/10.1016/j.bbadis.2014.04.007
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 285(17):13107–13120. https://doi.org/10.1074/jbc.M110.100420
Caccamo A, Maldonado MA, Majumder S, Medina DX, Holbein W, Magri A, Oddo S (2011) Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. J Biol Chem 286(11):8924–8932. https://doi.org/10.1074/jbc.M110.180638
Oddo S (2012) The role of mTOR signaling in Alzheimer disease. Front Biosci (Schol Ed) 4:941–952
Bove J, Martinez-Vicente M, Vila M (2011) Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 12(8):437–452. https://doi.org/10.1038/nrn3068
Gratuze M, Julien J, Petry FR, Morin F, Planel E (2017) Insulin deprivation induces PP2A inhibition and tau hyperphosphorylation in hTau mice, a model of Alzheimer’s disease-like tau pathology. Sci Rep 7:46359. https://doi.org/10.1038/srep46359
van der Harg JM, Eggels L, Bangel FN, Ruigrok SR, Zwart R, Hoozemans JJM, la Fleur SE, Scheper W (2017) Insulin deficiency results in reversible protein kinase A activation and tau phosphorylation. Neurobiol Dis 103:163–173. https://doi.org/10.1016/j.nbd.2017.04.005
Yang Y, Ma D, Wang Y, Jiang T, Hu S, Zhang M, Yu X, Gong CX (2013) Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J Alzheimers Dis 33(2):329–338. https://doi.org/10.3233/JAD-2012-121294
Chen Y, Run X, Liang Z, Zhao Y, Dai CL, Iqbal K, Liu F, Gong CX (2014) Intranasal insulin prevents anesthesia-induced hyperphosphorylation of tau in 3xTg-AD mice. Front Aging Neurosci 6:100. https://doi.org/10.3389/fnagi.2014.00100
De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ et al (2009) Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A 106(6):1971–1976. https://doi.org/10.1073/pnas.0809158106
Gispen WH, Biessels GJ (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23(11):542–549
Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV (2001) Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A 98(6):3561–3566. https://doi.org/10.1073/pnas.051634698
Author information
Authors and Affiliations
Contributions
EB, TC, and MP designed the study. EB, AT, FT, SC, and FDD performed the mouse experiments. TC and SG performed the behavioral tests. CR performed the electrophysiology experiments. EB, TC, MP, CG, and DAB analyzed and interpreted the data. EB and MP drafted the manuscript. All authors revised the manuscript critically for important intellectual content and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 141686 kb)
Rights and permissions
About this article
Cite this article
Barone, E., Tramutola, A., Triani, F. et al. Biliverdin Reductase-A Mediates the Beneficial Effects of Intranasal Insulin in Alzheimer Disease. Mol Neurobiol 56, 2922–2943 (2019). https://doi.org/10.1007/s12035-018-1231-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1231-5