Abstract
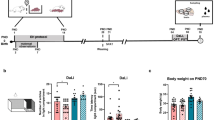
Previous studies showed that neonatal dexamethasone treatment (NDT) transiently impaired hippocampal function in male rats. Hippocampal estrogen receptors (ERs) participate in avoidance learning. As previous studies focused on males only, this study was aimed to investigate the NDT effects on the hippocampal function of female rats. Newborn Wistar female rats were subjected to a tapering dose of dexamethasone (0.5 mg, 0.3 mg, and 0.1 mg/kg, subcutaneously) from postnatal days 1 to 3 and were subjected to experiments at the age of 6 weeks (adolescence). Brain slice extracellular recording and the inhibitory avoidance (IA) test were used to evaluate the NDT effects on hippocampal function. The results showed that NDT completely blocked the hippocampal long-term potentiation (LTP) formation and IA learning of adolescents. The expression of hippocampal estrogen receptor alpha (ERα) was attenuated in NDT subjects. Reduced histone acetylation of the ERα gene was found, possibly explaining the reduced hippocampal ERα expression in NDT female rats. Suprafusion of estradiol (E2) partially restored the hippocampal LTP formation in adolescent NDT female rats. Coadministration of the histone deacetylase inhibitor trichostatin-A restored the hippocampal ERα expression, hippocampal LTP formation, and IA learning in adolescent NDT female rats. Collectively, these results suggested that NDT has an epigenetic modulation effect on the expression of hippocampal ERα, which is responsible for its adverse effect on hippocampal function.





Similar content being viewed by others
References
LeFlore JL, Salhab WA, Broyles RS, Engle WD (2002) Association of antenatal and postnatal dexamethasone exposure with outcomes in extremely low birth weight neonates. Pediatrics 110:275–279
Jefferies JM, Cooper T, Yam T, Clarke SC (2012) Pseudomonas aeruginosa outbreaks in the neonatal intensive care unit—a systematic review of risk factors and environmental sources. J Med Microbiol 61:1052–1061
Demauro SB, Dysart K, Kirpalani H (2014) Stopping the swinging pendulum of postnatal corticosteroid use. J Pediatr 164:9–11
Fauser A, Pohlandt F, Bartmann P, Gortner L (1993) Rapid increase of blood pressure in extremely low birth weight infants after a single dose of dexamethasone. Eur J Pediatr 152:354–356
Gordon PV, Young ML, Marshall DD (2001) Focal small bowel perforation: an adverse effect of early postnatal dexamethasone therapy in extremely low birth weight infants. J Perinatol 21:156–160
Gill AW, Warner G, Bull L (1996) Iatrogenic neonatal hypertrophic cardiomyopathy. Pediatr Cardiol 17:335–339
Verhaeghe J, Vanstapel F, Van Bree R, Van Herck E, Coopmans W (2007) Transient catabolic state with reduced IGF-I after antenatal glucocorticoids. Pediatr Res 62:295–300
Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ (2007) Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 32:256–266
Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WM (2008) Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res 61:106–112
Aisa B, Gil-Bea FJ, Marcos B, Tordera R, Lasheras B, Del Río J, Ramírez MJ (2009) Neonatal stress affects vulnerability of cholinergic neurons and cognition in the rat: involvement of the HPA axis. Psychoneuroendocrinology 34:1495–1505
McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348
de Vries WB, van den Borne P, Goldschmeding R, de Weger RA, Bal MP, van Bel F, van Oosterhout MF (2010) Neonatal dexamethasone treatment in the rat leads to kidney damage in adulthood. Pediatr Res 67:72–76
Bakker JM, Kavelaars A, Kamphuis PJ, Cobelens PM, van Vugt HH, van Bel F, Heijnen CJ (2000) Neonatal dexamethasone treatment increases susceptibility to experimental autoimmune disease in adult rats. J Immunol 165:5932–5937
le Cras TD, Markham NE, Morris KG, Ahrens CR, McMurtry IF, Abman SH (2000) Neonatal dexamethasone treatment increases the risk for pulmonary hypertension in adult rats. Am J Physiol Lung Cell Mol Physiol 278:L822–L829
Lee BH, Stoll BJ, McDonald SA, Higgins RD (2008) Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics 121:289–296
Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, Shankaran S, Finer NN et al (2009) Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 123:e430–e437
Barrington KJ (2001) Postnatal steroids and neurodevelopmental outcomes: a problem in the making. Pediatrics 107:1425–1426
Barrington KJ (2001) The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr 1(1)
Neal CR Jr, Weidemann G, Kabbaj M, Vázquez DM (2004) Effect of neonatal dexamethasone exposure on growth and neurological development in the adult rat. Am J Physiol Regul Integr Comp Physiol 287:R375–R385
Kamphuis PJ, Gardoni F, Kamal A, Croiset G, Bakker JM, Cattabeni F, Gispen WH, van Bel F et al (2003) Long-lasting effects of neonatal dexamethasone treatment on spatial learning and hippocampal synaptic plasticity: involvement of the NMDA receptor complex. FASEB J 17:911–913
Lin HJ, Huang CC, Hsu KS (2006) Effects of neonatal dexamethasone treatment on hippocampal synaptic function. Ann Neurol 59:939–951
Flagel SB, Vázquez DM, Watson SJ Jr, Neal CR Jr (2002) Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment, and stress response. Am J Physiol Regul Integr Comp Physiol 282:R55–R63
Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL (2011) Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology 214:141–154
Sherwin BB (2005) Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci 1052:3–10
Ko MC, Hung YH, Ho PY, Yang YL, Lu KT (2014) Neonatal glucocorticoid treatment increased depression-like behaviour in adult rats. Int J Neuropsychopharmacol 17:1995–2004
van der Heide-Jalving M, Kamphuis PJ, van der Laan MJ, Bakker JM, Wiegant VM, Heijnen CJ, Veen S, van Bel F (2003) Short- and long-term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone? Acta Paediatr 92:827–835
Zhu L, Li H, Tang J, Zhu J, Zhang Y (2012) Hyperoxia arrests alveolar development through suppression of histone deacetylases in neonatal rats. Pediatr Pulm 47:264–274
Foy MR, Baudry M, Diaz Brinton R, Thompson RF (2008) Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis 15:589–603
Prediger RD, Fernandes MS, Rial D, Wopereis S, Pereira VS, Bosse TS, Da Silva CB, Carradore RS et al (2008) Effects of acute administration of the hydroalcoholic extract of mate tea leaves (Ilex paraguariensis) in animal models of learning and memory. J Ethnopharmacol 120:465–473
Lucena GM, Prediger RD, Silva MV, Santos SN, Silva JF, Santos AR, Azevedo MS, Ferreira VM (2013) Ethanolic extract from bulbs of Cipura paludosa reduced long-lasting learning and memory deficits induced by prenatal methylmercury exposure in rats. Dev Cogn Neurosci 3:1–10
Ramos-Pratts K, Rosa-González D, Pérez-Acevedo NL, Cintrón-López D, Barreto-Estrada JL (2013) Sex-specific effect of the anabolic steroid, 17α-methyltestosterone, on inhibitory avoidance learning in periadolescent rats. Behav Process 99:73–80
Lee TI, Johnstone SE, Young RA (2006) Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc 1:729–748
McEwen BS, Alves SE (1999) Estrogen actions in the central nervous system. Endocr Rev 20:279–307
Duarte-Guterman P, Yagi S, Chow C, Galea LA (2015) Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm Behav 74:37–52
McEwen BS, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacol 41:3–23
Woolley CS, McEwen BS (1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12:2549–2554
Cui J, Shen Y, Li R (2013) Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol Med 19:197–209
Bean LA, Ianov L, Foster TC (2014) Estrogen receptors, the hippocampus, and memory. Neuroscientist 20:534–545
Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M (2001) Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A 98:13391–13395
Foy MR (2011) Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiol Learn Mem 95:134–144
Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P (1989) Steroid hormone receptors compete for factors that mediate their enhancer function. Cell 57:433–442
Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC (2013) Role of estrogen receptor α and β in preserving hippocampal function during aging. J Neurosci 33:2671–2683
Acknowledgments
We thank the Academic Paper Editing Clinic, National Taiwan Normal University (APEC of NTNU).
Funding
This study was supported by the Ministry of Science and Technology (MOST), Taiwan (103-2320-B-003-002 and 104-2320-B-003-00).
Author information
Authors and Affiliations
Contributions
KTL and YLY participated in the conception and design. CYC contributed to the electrophysiological experiments. HFC, MWYC, JLC, and JMJL participated in the ChIP experiment. HFC collected and assembled the data. HFC, MWYC, KTL, and YLY analyzed and interpreted the data. KTL and YLY wrote the manuscript and provided financial support. All authors corrected and approved the manuscript.
Corresponding authors
Ethics declarations
All procedures were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the local Institutional animal Care and Use Committee (IACUC) at the National Taiwan Normal University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chiu, HF., Chan, M.W.Y., Cheng, CY. et al. Neonatal Dexamethasone Treatment Suppresses Hippocampal Estrogen Receptor α Expression in Adolescent Female Rats. Mol Neurobiol 56, 2224–2233 (2019). https://doi.org/10.1007/s12035-018-1214-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1214-6