Abstract
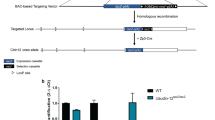
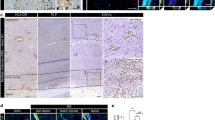
It is important to understand the molecular mechanisms of barrier disruption in the central nervous system (CNS) of patients with multiple sclerosis (MS). The purpose of the present study was to clarify whether claudin-11 is involved in the disruption of two endothelial barriers (blood-brain barrier (BBB) and blood-spinal cord barrier (BSCB)) and two epithelial barriers (blood-arachnoid barrier (BAB) and blood-CSF barrier (BCSFB)) in the CNS in MS. Immunohistochemical analysis revealed that, in both normal human and mouse, claudin-11 is co-localized with claudin-5 in the brain and spinal cord capillaries. The absolute protein expression level of claudin-11 was nearly equal to that of claudin-5 in rat brain capillaries, but was 2.81-fold greater in human brain capillaries. The protein expressions of claudin-11 were significantly downregulated in the brain and spinal cord capillaries of an MS patient and experimental autoimmune encephalomyelitis (EAE) mice. Specific downregulation of claudin-11 with siRNA significantly increased the transfer of membrane-impermeable FITC-dextran across human brain capillary endothelial cell (hCMEC/D3) monolayer. As for the epithelial barrier, claudin-11 protein expression was not decreased in choroid plexus epithelial cells forming the BCSFB in EAE mice, whereas it was decreased in brain and spinal cord meninges that form the BAB. Specific downregulation of claudin-11 with siRNA in a rat choroid plexus epithelial cell (TR-CSFB) monolayer significantly increased the permeability of FITC-dextran. In conclusion, our present findings indicate that claudin-11 expression at the BBB, BSCB, and BAB, but not the BCSFB, is downregulated in multiple sclerosis, impairing the functional integrity of these barriers.






Similar content being viewed by others
Abbreviations
- BAB:
-
Blood-arachnoid barrier
- BBB:
-
Blood-brain barrier
- BCSFB:
-
Blood-cerebrospinal fluid barrier
- B-cap:
-
Brain capillary
- BSCB:
-
Blood-spinal cord barrier
- DDA:
-
Data-dependent acquisition
- DHT:
-
Dihydrotestosterone
- EAE:
-
Experimental autoimmune encephalomyelitis
- ER-TR7:
-
A meningeal marker
- Glut1:
-
Glucose transporter 1
- hCMEC/D3:
-
Human cerebral microvascular endothelial cell line
- LC-MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- MS:
-
Multiple sclerosis
- PRM:
-
Parallel reaction monitoring
- QTAP:
-
Quantitative targeted absolute proteomics
- SC-cap:
-
Spinal cord capillary
- SRM:
-
Selected reaction monitoring
- TM-BBB:
-
Mouse brain capillary endothelial cell line
- TR-CSFB:
-
Rat choroid plexus epithelial cell line
References
Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P et al (1990) Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain 113(Pt 5):1477–1489
Errede M, Girolamo F, Ferrara G, Strippoli M, Morando S, Boldrin V, Rizzi M, Uccelli A et al (2012) Blood-brain barrier alterations in the cerebral cortex in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol 71(10):840–854. https://doi.org/10.1097/NEN.0b013e31826ac110
Kooij G, Kopplin K, Blasig R, Stuiver M, Koning N, Goverse G, van der Pol SM, van Het Hof B et al (2014) Disturbed function of the blood-cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathol 128(2):267–277. https://doi.org/10.1007/s00401-013-1227-1
Hellani A, Ji J, Mauduit C, Deschildre C, Tabone E, Benahmed M (2000) Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/claudin 11 in mouse testis. Endocrinology 141(8):3012–3019. https://doi.org/10.1210/endo.141.8.7625
Tiwari-Woodruff SK, Buznikov AG, Vu TQ, Micevych PE, Chen K, Kornblum HI, Bronstein JM (2001) OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes. J Cell Biol 153(2):295–305
Brochner CB, Holst CB, Mollgard K (2015) Outer brain barriers in rat and human development. Front Neurosci 9:75. https://doi.org/10.3389/fnins.2015.00075
Romanitan MO, Popescu BO, Spulber S, Bajenaru O, Popescu LM, Winblad B, Bogdanovic N (2010) Altered expression of claudin family proteins in Alzheimer's disease and vascular dementia brains. J Cell Mol Med 14(5):1088–1100. https://doi.org/10.1111/j.1582-4934.2009.00999.x
Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B (2001) Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett 307(2):77–80
Florin A, Maire M, Bozec A, Hellani A, Chater S, Bars R, Chuzel F, Benahmed M (2005) Androgens and postmeiotic germ cells regulate claudin-11 expression in rat Sertoli cells. Endocrinology 146(3):1532–1540. https://doi.org/10.1210/en.2004-0834
Kaitu'u-Lino TJ, Sluka P, Foo CF, Stanton PG (2007) Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction 133(6):1169–1179. https://doi.org/10.1530/REP-06-0385
Tomassini V, Onesti E, Mainero C, Giugni E, Paolillo A, Salvetti M, Nicoletti F, Pozzilli C (2005) Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry 76(2):272–275. https://doi.org/10.1136/jnnp.2003.033324
Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A, Starmer J, Wilson EM et al (2016) Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun 7:11350. https://doi.org/10.1038/ncomms11350
Prockop LD, Naidu KA, Binard JE, Ransohoff J (1995) Selective permeability of [3H]-D-mannitol and [14C]-carboxyl-inulin across the blood-brain barrier and blood-spinal cord barrier in the rabbit. J Spinal Cord Med 18(4):221–226
Schlaeger R, Papinutto N, Zhu AH, Lobach IV, Bevan CJ, Bucci M, Castellano A, Gelfand JM et al (2015) Association between thoracic spinal cord gray matter atrophy and disability in multiple sclerosis. JAMA Neurol 72(8):897–904. https://doi.org/10.1001/jamaneurol.2015.0993
Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV (2012) Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 32(10):1841–1852. https://doi.org/10.1038/jcbfm.2012.113
Schlager C, Korner H, Krueger M, Vidoli S, Haberl M, Mielke D, Brylla E, Issekutz T et al (2016) Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530(7590):349–353. https://doi.org/10.1038/nature16939
Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A et al (2005) Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 19(13):1872–1874. https://doi.org/10.1096/fj.04-3458fje
Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T (2011) Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem 117(2):333–345
Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T (2013) Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci 102(9):3343–3355. https://doi.org/10.1002/jps.23575
Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, Sekine Y, Uchida Y et al (2008) Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res 25(6):1469–1483
Uchida Y, Ohtsuki S, Kamiie J, Terasaki T (2011) Blood-brain barrier (BBB) pharmacoproteomics: reconstruction of in vivo brain distribution of 11 P-glycoprotein substrates based on the BBB transporter protein concentration, in vitro intrinsic transport activity, and unbound fraction in plasma and brain in mice. J Pharmacol Exp Ther 339(2):579–588. https://doi.org/10.1124/jpet.111.184200
Morgan L, Shah B, Rivers LE, Barden L, Groom AJ, Chung R, Higazi D, Desmond H et al (2007) Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience 147(3):664–673. https://doi.org/10.1016/j.neuroscience.2007.04.051
Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T (2008) mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem 104(1):147–154. https://doi.org/10.1111/j.1471-4159.2007.05008.x
Gong Y, Renigunta V, Zhou Y, Sunq A, Wang J, Yang J, Renigunta A, Baker LA et al (2015) Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Mol Biol Cell 26(24):4333–4346. https://doi.org/10.1091/mbc.E15-06-0422
Hou J, Renigunta A, Yang J, Waldegger S (2010) Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A 107(42):18010–18015. https://doi.org/10.1073/pnas.1009399107
Uchida Y, Zhang Z, Tachikawa M, Terasaki T (2015) Quantitative targeted absolute proteomics of rat blood-cerebrospinal fluid barrier transporters: comparison with a human specimen. J Neurochem 134(6):1104–1115. https://doi.org/10.1111/jnc.13147
Kolias AG, Chari A, Santarius T, Hutchinson PJ (2014) Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 10(10):570–578. https://doi.org/10.1038/nrneurol.2014.163
Thorne RG (2014) Primer on central nervous system structure/function and the vasculature, ventricular system, and fluids of the brai. In: Hammarlund-Udenaes M, de Lange E, Thorne RG (eds) Drug delivery to the brain - physiological concepts, methodologies and approaches. Springer, pp. 685–707
Lui WY, Lee WM (2009) Molecular mechanisms by which hormones and cytokines regulate cell junction dynamics in the testis. J Mol Endocrinol 43(2):43–51. https://doi.org/10.1677/JME-08-0174
Yan HH, Mruk DD, Lee WM, Cheng CY (2008) Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J 22(6):1945–1959. https://doi.org/10.1096/fj.06-070342
Lanz TV, Becker S, Osswald M, Bittner S, Schuhmann MK, Opitz CA, Gaikwad S, Wiestler B et al (2013) Protein kinase Cbeta as a therapeutic target stabilizing blood-brain barrier disruption in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 110(36):14735–14740. https://doi.org/10.1073/pnas.1302569110
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. https://doi.org/10.1083/jcb.200302070
Acknowledgements
We thank Prof Koji Fukunaga (Graduate School of Pharmaceutical Sciences, Tohoku University, Japan) for making available the confocal laser-scanning microscope, and A. Niitomi and N. Handa for their secretarial assistance.
Funding
This study was supported in part by three Grants-in-Aids from the Japanese Society for the Promotion of Science (JSPS) for Challenging Exploratory Research (KAKENHI 16K15475), Young Scientists (A) (KAKENHI 16H06218), and Scientific Research (B) (KAKENHI 17H04004), and was also supported in part by the Nakatomi Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Tetsuya Terasaki and Sumio Ohtsuki are full professors at Tohoku University and Kumamoto University, and are also directors of Proteomedix Frontiers Co., Ltd. This study was not supported by Proteomedix Frontiers Co., Ltd., and their positions at Proteomedix Frontiers Co., Ltd., did not influence the design of the study, the collection of data, the analysis or interpretation of data, the decision to submit the manuscript for publication, or writing of the manuscript. There were no financial conflicts. The other authors declare no competing interests.
Electronic Supplementary Material
Supplemental data including the experimental data and the details of the materials and methods is available at Molecular Neurobiology online.
ESM 1
(PDF 700 kb)
Rights and permissions
About this article
Cite this article
Uchida, Y., Sumiya, T., Tachikawa, M. et al. Involvement of Claudin-11 in Disruption of Blood-Brain, -Spinal Cord, and -Arachnoid Barriers in Multiple Sclerosis. Mol Neurobiol 56, 2039–2056 (2019). https://doi.org/10.1007/s12035-018-1207-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1207-5