Abstract
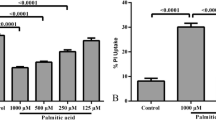
Obesity is associated with an increase in the brain levels of saturated free fatty acids, such as palmitic acid (PA). Previous studies have shown that PA exerts proinflammatory actions and reduces cell viability in astrocyte cultures. In this study, we have assessed whether an alteration in autophagy is involved in the effects of PA on astrocytes. Primary astrocytes were obtained from the cerebral cortex of male and female CD1 mouse pups and were incubated for 4.5 or 24 h with 250–500 μM PA. PA increased the levels of LC3-II, an autophagosome marker, and reduced LC3-II flux in astrocytes, suggesting a blockade of autophagy. This effect was observed both after 4.5 and 24 h of treatment with PA. PA had additional effects after treatment for 24 h, increasing the expression of proinflammatory cytokines, decreasing cell viability, and increasing the levels of an endoplasmic reticulum stress marker. In addition, PA decreased the expression of estrogen receptors, but only in female astrocytes. However, the treatment with estradiol, estrogen receptor agonists, or inhibitor of estradiol synthesis did not counteract the action of PA on cell viability. Rapamycin, an autophagy inducer, was unable to prevent the effect of PA on cell viability. In addition, hydroxychloroquine, an autophagy blocker, did not cause per se astrocyte death. These findings suggest that the effect of PA on autophagy is not sufficient to induce astrocyte loss, which is only observed when prolonged PA treatment causes other alterations in astrocytes, such as increased inflammation and endoplasmic reticulum stress.









Similar content being viewed by others
References
World Health Organization (2017) Obesity and overweight. WHO Media Center. http://www.who.int/mediacentre/factsheets/fs311/en. Accessed 19 March 2018
Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC (2005) The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol 5:23. https://doi.org/10.1186/1471-2377-5-23
Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD et al (2010) Brain structure and obesity. Hum Brain Mapp 31:353–364. https://doi.org/10.1002/hbm.20870
Gunstad J, Paul R, Cohen R, Tate D, Spitznagel M, Gordon E (2007) Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry 48:57–61. https://doi.org/10.1016/j.comppsych.2006.05.001
Cheke LG, Bonnici HM, Clayton NS, Simons JS (2017) Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia 96:137–149. https://doi.org/10.1016/j.neuropsychologia.2017.01.013
Profenno LA, Porsteinsson AP, Faraone SV (2010) Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 67:505–512. https://doi.org/10.1016/j.biopsych.2009.02.013
Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, Turner N (2013) Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56:1129–1139. https://doi.org/10.1007/s00125-013-2846-8
Stranahan AM, Hao S, Dey A, Yu X, Baban B (2016) Blood–brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab 36:2108–2121. https://doi.org/10.1177/0271678X16642233
Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F (2002) A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 112:803–814. https://doi.org/10.1016/S0306-4522(02)00123-9
Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP (2008) Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18:1085–1088. https://doi.org/10.1002/hipo.20470
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95:2409–2415. https://doi.org/10.1172/JCI117936
Thaler J, Yi C, Schur E, Guyenet S, Hwang B, Dietrich M, Zhao X, Sarruf D et al (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122:153–162. https://doi.org/10.1172/JCI59660
Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D (2008) Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73. https://doi.org/10.1016/j.cell.2008.07.043
Almeida-Suhett CP, Graham A, Chen Y, Deuster P (2017) Behavioral changes in male mice fed a high-fat diet are associated with IL-1β expression in specific brain regions. Physiol Behav 169:130–140. https://doi.org/10.1016/j.physbeh.2016.11.016
Franssen R, Monajemi H, Stroes ES, Kastelein JJ (2011) Obesity and dyslipidemia. Med Clin North Am 95:893–902. https://doi.org/10.1016/j.mcna.2011.06.003
Smith QR, Nagura H (2001) Fatty acid uptake and incorporation in brain: studies with the perfusion model. J Mol Neurosci 16:167–172; discussion 215–21. https://doi.org/10.1385/JMN:16:2-3:167
Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R et al (2014) Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep 9:633–645. https://doi.org/10.1016/j.celrep.2014.09.025
Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J et al (2010) Increased brain fatty acid uptake in metabolic syndrome. Diabetes 59:2171–2177. https://doi.org/10.2337/db09-0138
Yanguas-Casás N, Crespo-Castrillo A, de Ceballos ML, Chowen JA, Azcoitia I, Arevalo MA, Garcia-Segura LM (2018) Sex differences in the phagocytic and migratory activity of microglia and their impairment by palmitic acid. Glia 66:522–537. https://doi.org/10.1002/glia.23263
Patil S, Melrose J, Chan C (2007) Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur J Neurosci 26:2131–2141. https://doi.org/10.1111/j.1460-9568.2007.05797.x
Wang Z, Liu D, Wang J, Liu S, Gao M, Ling EA, Hao A (2012) Cytoprotective effects of melatonin on astroglial cells subjected to palmitic acid treatment in vitro. J Pineal Res 52:253–264. https://doi.org/10.1111/j.1600-079X.2011.00952.x
Wong KL, Wu YR, Cheng KS, Chan P, Cheung CW, Lu DY, Su TH, Liu ZM et al (2014) Palmitic acid-induced lipotoxicity and protection by (+)-catechin in rat cortical astrocytes. Pharmacol Reports 66:1106–1113. https://doi.org/10.1016/j.pharep.2014.07.009
Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ (2012) Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem 120:1060–1071. https://doi.org/10.1111/j.1471-4159.2012.07660.x
Freire-Regatillo A, Argente-Arizón P, Argente J, García-Segura LM, Chowen JA (2017) Non-neuronal cells in the hypothalamic adaptation to metabolic signals. Front Endocrinol (Lausanne) 8:51. https://doi.org/10.3389/fendo.2017.00051
Blázquez C, Galve-Roperh I, Guzmán M (2000) De novo-synthesized ceramide signals apoptosis in astrocytes via extracellular signal-regulated kinase. FASEB J 14:2315–2322. https://doi.org/10.1096/fj.00-0122com
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330:1344–1348. https://doi.org/10.1126/science.1193497
Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT et al (2017) Molecular definitions of autophagy and related processes. EMBO J 36:1811–1836. https://doi.org/10.15252/embj.201796697
Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D et al (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25:1025–1040. https://doi.org/10.1128/MCB.25.3.1025
Ravanan P, Srikumar IF, Talwar P (2017) Autophagy: the spotlight for cellular stress responses. Life Sci 188:53–67. https://doi.org/10.1016/j.lfs.2017.08.029
Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS (2011) Fatty acids suppress autophagic turnover in B-cells. J Biol Chem 286:42534–42544. https://doi.org/10.1074/jbc.M111.242412
González-Rodríguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillón J, Lo Iacono O et al (2014) Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis 5:e1179. https://doi.org/10.1038/cddis.2014.162
Mir SUR, George NM, Zahoor L, Harms R, Guinn Z, Sarvetnick NE (2015) Inhibition of autophagic turnover in β-cells by fatty acids and glucose leads to apoptotic cell death. J Biol Chem 290:6071–6085. https://doi.org/10.1074/jbc.M114.605345
Trudeau KM, Colby AH, Zeng J, Las G, Feng JH, Grinstaff MW, Shirihai OS (2016) Lysosome acidification by photoactivated nanoparticles restores autophagy under lipotoxicity. J Cell Biol 214:25–34. https://doi.org/10.1083/jcb.201511042
Liu D, Ke Z, Luo J (2017) Thiamine deficiency and neurodegeneration: the interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol Neurobiol 54:5440–5448. https://doi.org/10.1007/s12035-016-0079-9
Qian M, Fang X, Wang X (2017) Autophagy and inflammation. Clin Transl Med 6:24. https://doi.org/10.1186/s40169-017-0154-5
Palmer BF, Clegg DJ (2015) The sexual dimorphism of obesity. Mol Cell Endocrinol 402:113–119. https://doi.org/10.1016/j.mce.2014.11.029
Chowen JA, Argente-Arizón P, Freire-Regatillo A, Argente J (2018) Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Front Neuroendocrinol 48:3–12. https://doi.org/10.1016/j.yfrne.2017.05.003
Ruiz-Palmero I, Simon-Areces J, Garcia-Segura LM, Arevalo MA (2011) Notch/Neurogenin 3 signalling is involved in the neuritogenic actions of oestradiol in developing hippocampal neurones. J Neuroendocrinol 23:355–364. https://doi.org/10.1111/j.1365-2826.2011.02110.x
Grassi D, Bellini MJ, Acaz-Fonseca E, Panzica G, Garcia-Segura LM (2013) Estradiol and testosterone regulate arginine-vasopressin expression in SH-SY5Y human female neuroblastoma cells through estrogen receptors-α and -β. Endocrinology 154:2092–2100. https://doi.org/10.1210/en.2012-2137
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45–e445. https://doi.org/10.1093/nar/29.9.e45
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515. https://doi.org/10.1023/B:BILE.0000019559.84305.47
Portovedo M, Ignacio-Souza LM, Bombassaro B, Coope A, Reginato A, Razolli DS, Torsoni MA, Torsoni AS et al (2015) Saturated fatty acids modulate autophagy’s proteins in the hypothalamus. PLoS One 10:e0119850. https://doi.org/10.1371/journal.pone.0119850
Yamato M, Shiba T, Yoshida M, Ide T, Seri N, Kudou W, Kinugawa S, Tsutsui H (2007) Fatty acids increase the circulating levels of oxidative stress factors in mice with diet-induced obesity via redox changes of albumin. FEBS J 274:3855–3863. https://doi.org/10.1111/j.1742-4658.2007.05914.x
Alsahli A, Kiefhaber K, Gold T, Muluke M, Jiang H, Cremers S, Schulze-Späte U (2016) Palmitic acid reduces circulating bone formation markers in obese animals and impairs osteoblast activity via C16-ceramide accumulation. Calcif Tissue Int 98:511–519. https://doi.org/10.1007/s00223-015-0097-z
Benito-Cuesta I, Diez H, Ordoñez L, Wandosell F (2017) Assessment of autophagy in neurons and brain tissue. Cells 6:25. https://doi.org/10.3390/cells6030025
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata JI et al (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163. https://doi.org/10.1016/j.cell.2007.10.035
Blommaart EF, Luiken JJ, Blommaart PJ, Van Woerkom GM, Meijer AJ (1995) Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem 270:2320–2326. https://doi.org/10.1074/jbc.270.5.2320
Fitzwalter BE, Thorburn A (2015) Recent insights into cell death and autophagy. FEBS J 282:4279–4288. https://doi.org/10.1111/febs.13515
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389. https://doi.org/10.1038/sj.cdd.4401373
González-Giraldo Y, Garcia-Segura LM, Echeverria V, Barreto GE (2018) Tibolone preserves mitochondrial functionality and cell morphology in astrocytic cells treated with palmitic acid. Mol Neurobiol 55:4453–4462. https://doi.org/10.1007/s12035-017-0667-3
Liu L, Martin R, Chan C (2013) Palmitate-activated astrocytes via serine palmitoyltransferase increase BACE1 in primary neurons by sphingomyelinases. Neurobiol Aging 34:540–550. https://doi.org/10.1016/j.neurobiolaging.2012.05.017
Su X, Chu Y, Kordower JH, Li B, Cao H, Huang L, Nishida M, Song L et al (2015) PGC-1α promoter methylation in Parkinson’s disease. PLoS One 10:e0134087. https://doi.org/10.1371/journal.pone.0134087
Frago LM, Canelles S, Freire-Regatillo A, Argente-Arizón P, Barrios V, Argente J, Garcia-Segura LM, Chowen JA (2017) Estradiol uses different mechanisms in astrocytes from the hippocampus of male and female rats to protect against damage induced by palmitic acid. Front Mol Neurosci 10:330. https://doi.org/10.3389/fnmol.2017.00330
Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM (2011) Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ 2:7. https://doi.org/10.1186/2042-6410-2-7
Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HEW et al (2012) Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 37:1688–1699. https://doi.org/10.1016/j.psyneuen.2012.02.018
Astiz M, Acaz-Fonseca E, Garcia-Segura LM (2014) Sex differences and effects of estrogenic compounds on the expression of inflammatory molecules by astrocytes exposed to the insecticide dimethoate. Neurotox Res 25:271–285. https://doi.org/10.1007/s12640-013-9417-0
De Marinis E, Acaz-Fonseca E, Arevalo MA, Ascenzi P, Fiocchetti M, Marino M, Garcia-Segura LM (2013) 17β-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor β-mediated neuroglobin up-regulation. J Neuroendocrinol 25:260–270. https://doi.org/10.1111/jne.12007
Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM (2003) Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci 1007:298–305. https://doi.org/10.1196/annals.1286.028
Pilitsis JG, Diaz FG, O’Regan MH, Phillis JW (2002) Differential effects of phospholipase inhibitors on free fatty acid efflux in rat cerebral cortex during ischemia-reperfusion injury. Brain Res 951:96–106. https://doi.org/10.1016/S0006-8993(02)03142-6
Fraser T, Tayler H, Love S (2010) Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochem Res 35:503–513. https://doi.org/10.1007/s11064-009-0087-5
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM et al (2009) Autophagy regulates lipid metabolism. Nature 458:1131–1135. https://doi.org/10.1038/nature07976
Singh R, Cuervo AM (2012) Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol 2012:282041–282012. https://doi.org/10.1155/2012/282041
Mei S, Ni HM, Manley S, Bockus A, Kassel KM, Luyendyk JP, Copple BL, Ding WX (2011) Differential roles of unsaturated and saturated fatty acids on autophagy and apoptosis in hepatocytes. J Pharmacol Exp Ther 339:487–498. https://doi.org/10.1124/jpet.111.184341
Cnop M, Abdulkarim B, Bottu G, Cunha DA, Igoillo-Esteve M, Masini M, Turatsinze JV, Griebel T et al (2014) RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes 63:1978–1993. https://doi.org/10.2337/db13-1383
Akoumi A, Haffar T, Mousterji M, Kiss RS, Bousette N (2017) Palmitate mediated diacylglycerol accumulation causes endoplasmic reticulum stress, Plin2 degradation, and cell death in H9C2 cardiomyoblasts. Exp Cell Res 354:85–94. https://doi.org/10.1016/j.yexcr.2017.03.032
Jung TW, Hong HC, Hwang HJ, Yoo HJ, Baik SH, Choi KM (2015) C1q/TNF-related protein 9 (CTRP9) attenuates hepatic steatosis via the autophagy-mediated inhibition of endoplasmic reticulum stress. Mol Cell Endocrinol 417:131–140. https://doi.org/10.1016/j.mce.2015.09.027
Yoshii SR, Mizushima N (2017) Monitoring and measuring autophagy. Int J Mol Sci 18:1–13. https://doi.org/10.3390/ijms18091865
Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614. https://doi.org/10.1083/jcb.200507002
Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EMC, Isaacs A, Brech A et al (2007) Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol 179:485–500. https://doi.org/10.1083/jcb.200702115
B’Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G et al (2013) The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 41:7683–7699. https://doi.org/10.1093/nar/gkt563
Song C, Mitter SK, Qi X, Beli E, Rao HV, Ding J, Ip CS, Gu H et al (2017) Oxidative stress-mediated NFκB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS One 12:1–26. https://doi.org/10.1371/journal.pone.0171940
Acknowledgements
We thank Elisa Baides Rosell and Nieves Lopez Andradas for excellent technical assistance.
Funding
This work was supported by grants from Ministerio de Economía, Industria y Competitividad (MINECO), Spain (grant numbers BFU2014–51836-C2-1-R and BFU2017-82754-R); Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Instituto de Salud Carlos III, Madrid, Spain; and Fondos FEDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the procedures involving animals used in this study followed the European Commission guidelines (2010/63/UE) and Spanish regulation (RD 53/2013) on the protection of animals for experimental use. These procedures were approved by our institutional animal care and use committee (Comité de Ética de Experimentación Animal del Instituto Cajal) and the Consejería del Medio Ambiente y Territorio (Comunidad de Madrid, PROEX 200/14).
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 1536 kb)
Rights and permissions
About this article
Cite this article
Ortiz-Rodriguez, A., Acaz-Fonseca, E., Boya, P. et al. Lipotoxic Effects of Palmitic Acid on Astrocytes Are Associated with Autophagy Impairment. Mol Neurobiol 56, 1665–1680 (2019). https://doi.org/10.1007/s12035-018-1183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1183-9