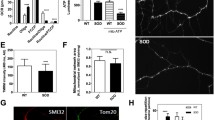
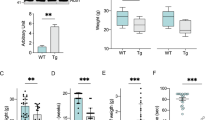
Abstract
Amyotrophic lateral sclerosis (ALS) is an adult-onset fatal neurodegenerative disease characterized by muscle wasting, weakness, and spasticity due to a progressive degeneration of cortical, brainstem, and spinal motor neurons. The etiopathological causes are still largely obscure, although astrocytes definitely play a role in neuronal damage. Several mechanisms have been proposed to concur to neurodegeneration in ALS, including mitochondrial dysfunction. We have previously shown profound modifications of glutamate release and presynaptic plasticity in the spinal cord of the SOD1G93A mouse model of ALS. In this work, we characterized, for the first time, the aerobic metabolism in two specific compartments actively involved in neurotransmission (i.e. the presynaptic district, using purified synaptosomes, and the perisynaptic astrocyte processes, using purified gliosomes) in SOD1G93A mice at different stages of the disease. ATP/AMP ratio was lower in synaptosomes isolated from the spinal cord, but not from other brain areas, of SOD1G93A vs. control mice. The energy impairment was linked to altered oxidative phosphorylation (OxPhos) and increment of lipid peroxidation. These metabolic dysfunctions were present during disease progression, starting at the very pre-symptomatic stages, and did not depend on a different number of mitochondria or a different expression of OxPhos proteins. Conversely, gliosomes showed a reduction of the ATP/AMP ratio only at the late stages of the disease and an increment of oxidative stress also in the absence of a significant decrement in OxPhos activity. Data suggest that the presynaptic neuronal moiety plays a pivotal role for synaptic energy metabolism dysfunctions in ALS. Changes in the perisynaptic compartment seem subordinated to neuronal damage.






Similar content being viewed by others
References
Brown RH Jr (1995) Superoxide dismutase in familial amyotrophic lateral sclerosis: models for gain of function. Curr Opin Neurobiol 5:841–846 doi: 0959-4388(95)80114-6 [pii]
Eisen A (2009) Amyotrophic lateral sclerosis—evolutionary and other perspectives. Muscle Nerve 40:297–304. https://doi.org/10.1002/mus.21404
Rowland LP, Shneider NA (2001) Amyotrophic lateral sclerosis. N Engl J Med 344:1688–1700. https://doi.org/10.1056/NEJM200105313442207
Turner MR, Bowser R, Bruijn L, Dupuis L, Ludolph A, McGrath M, Manfredi G, Maragakis N et al (2013) Mechanisms, models and biomarkers in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener 14:19–32. https://doi.org/10.3109/21678421.2013.778554
Lu H, Le WD, Xie Y-Y, Wang X-P (2016) Current therapy of drugs in amyotrophic lateral sclerosis. Curr Neuropharmacol 14:314–321
Abe K, Aoki M, Tsuji S, Itoyama Y, Sobue G, Togo M, Hamada C, Tanaka M et al (2017) Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 16:505–512. https://doi.org/10.1016/S1474-4422(17)30115-1
Andersen PM, Al-Chalabi A (2011) Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol 7:603–615. https://doi.org/10.1038/nrneurol.2011.150
Kaur SJ, McKeown SR, Rashid S (2016) Mutant SOD1 mediated pathogenesis of amyotrophic lateral sclerosis. Gene 577:109–118. https://doi.org/10.1016/j.gene.2015.11.049
Philips T, Rothstein JD (2015) Rodent models of amyotrophic lateral sclerosis. Curr Protoc Pharmacol 69:5.67.1–5.6721. https://doi.org/10.1002/0471141755.ph0567s69
Johnson FO, Atchison WD (2009) The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 30(5):761–765. https://doi.org/10.1016/j.neuro.2009.07.010
Callaghan B, Feldman D, Gruis K, Feldman E (2011) The association of exposure to lead, mercury, and selenium and the development of amyotrophic lateral sclerosis and the epigenetic implications. Neurodegener Dis 8:1–8. https://doi.org/10.1159/000315405
Cleveland DW, Bruijn LI, Wong PC, Marszalek JR, Vechio JD, Lee MK, Xu XS, Borchelt DR, Sisodia SS, Price DL (1996) Mechanisms of selective motor neuron death in transgenic mouse models of motor neuron disease. Neurology 47:S54–61–2, 47, 54S, 62S.
Morrison BM, Morrison JH (1999) Amyotrophic lateral sclerosis associated with mutations in superoxide dismutase: a putative mechanism of degeneration. Brain Res Brain Res Rev 29:121–135
Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W (2006) The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta - Mol Basis Dis 1762:1068–1082. https://doi.org/10.1016/j.bbadis.2006.05.002
Matus S, Valenzuela V, Medinas DB, Hetz C (2013) ER dysfunction and protein folding stress in ALS. Int J Cell Biol 2013:1–12. https://doi.org/10.1155/2013/674751
Droppelmann CA, Campos-Melo D, Ishtiaq M, Volkening K, Strong MJ (2014) RNA metabolism in ALS: when normal processes become pathological. Amyotroph Lateral Scler Front Degener 15:321–336. https://doi.org/10.3109/21678421.2014.881377
Tan W, Pasinelli P, Trotti D (2014) Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis. Biochim Biophys Acta - Mol Basis Dis 1842:1295–1301. https://doi.org/10.1016/j.bbadis.2014.02.009
Peters OM, Ghasemi M, Brown RH (2015) Emerging mechanisms of molecular pathology in ALS. J Clin Invest 125:1767–1779. https://doi.org/10.1172/JCI71601
King AE, Woodhouse A, Kirkcaldie MTK, Vickers JC (2016) Excitotoxicity in ALS: overstimulation, or overreaction? Exp Neurol 275:162–171. https://doi.org/10.1016/j.expneurol.2015.09.019
Carrì MT, Valle C, Bozzo F, Cozzolino M (2015) Oxidative stress and mitochondrial damage: importance in non-SOD1 ALS. Front Cell Neurosci 9:41. https://doi.org/10.3389/fncel.2015.00041
Dupuis L, Pradat PF, Ludolph AC, Loeffler JP (2011) Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol 10:75–82. https://doi.org/10.1016/S1474-4422(10)70224-6
Browne SE, Yang L, DiMauro JP et al (2006) Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol Dis 22:599–610. https://doi.org/10.1016/j.nbd.2006.01.001
Mattiazzi M, D’Aurelio M, Gajewski CD et al (2002) Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem 277:29626–29633. https://doi.org/10.1074/jbc.M203065200
Ferri A, Cozzolino M, Crosio C, Nencini M, Casciati A, Gralla EB, Rotilio G, Valentine JS et al (2006) Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc Natl Acad Sci U S A 103:13860–13865. https://doi.org/10.1073/pnas.0605814103
Kawamata H, Manfredi G (2010) Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech Ageing Dev 131:517–526. https://doi.org/10.1016/j.mad.2010.05.003
Ferri A, Nencini M, Cozzolino M, Carrara P, Moreno S, Carrì MT (2008) Inflammatory cytokines increase mitochondrial damage in motoneuronal cells expressing mutant SOD1. Neurobiol Dis 32:454–460. https://doi.org/10.1016/j.nbd.2008.08.004
Cozzolino M, Pesaresi MG, Amori I, Crosio C, Ferri A, Nencini M, Carrì MT (2009) Oligomerization of mutant SOD1 in mitochondria of motoneuronal cells drives mitochondrial damage and cell toxicity. Antioxid Redox Signal 11:1547–1558. https://doi.org/10.1089/ars.2009.2545
Pesaresi MG, Amori I, Giorgi C, Ferri A, Fiorenzo P, Gabanella F, Salvatore AM, Giorgio M et al (2011) Mitochondrial redox signalling by p66Shc mediates ALS-like disease through Rac1 inactivation. Hum Mol Genet 20:4196–4208. https://doi.org/10.1093/hmg/ddr347
Cozzolino M, Ferri A, Ferraro E, Rotilio G, Cecconi F, Carrì MT (2006) Apaf1 mediates apoptosis and mitochondrial damage induced by mutant human SOD1s typical of familial amyotrophic lateral sclerosis. Neurobiol Dis 21:69–79. https://doi.org/10.1016/j.nbd.2005.06.010
Sasaki S, Iwata M (1999) Ultrastructural change of synapses of Betz cells in patients with amyotrophic lateral sclerosis. Neurosci Lett 268:29–32
Menzies FM, Ince PG, Shaw PJ (2002) Mitochondrial involvement in amyotrophic lateral sclerosis. Neurochem Int 40:543–551
Martin LJ (2011) Mitochondrial pathobiology in ALS. J Bioenerg Biomembr 43:569–579. https://doi.org/10.1007/s10863-011-9395-y
Coussee E, De Smet P, Bogaert E et al (2011) G37R SOD1 mutant alters mitochondrial complex I activity, Ca(2+) uptake and ATP production. Cell Calcium 49:217–225. https://doi.org/10.1016/j.ceca.2011.02.004
Ilieva H, Polymenidou M, Cleveland DW (2009) Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187:761–772. https://doi.org/10.1083/jcb.200908164
Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song SW, Likhite S et al (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29:824–828. https://doi.org/10.1038/nbt.1957
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci 22:208–215. https://doi.org/10.1016/S0166-2236(98)01349-6
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. https://doi.org/10.1016/j.tins.2009.05.001
Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG (2017) Astrocytic control of synaptic function. Philos Trans R Soc B Biol Sci 372:20160154. https://doi.org/10.1098/rstb.2016.0154
Allen NJ, Barres BA (2005) Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol 15:542–548. https://doi.org/10.1016/j.conb.2005.08.006
Bilsland LG, Nirmalananthan N, Yip J, Greensmith L, Duchen MR (2008) Expression of mutant SOD1 G93A in astrocytes induces functional deficits in motoneuron mitochondria. J Neurochem 107:1271–1283. https://doi.org/10.1111/j.1471-4159.2008.05699.x
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10:615–622. https://doi.org/10.1038/nn1876
Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de Leon A, Robinson KM, Mason RP et al (2008) Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci 28:4115–4122. https://doi.org/10.1523/JNEUROSCI.5308-07.2008
Raiteri L, Stigliani S, Zappettini S, Mercuri NB, Raiteri M, Bonanno G (2004) Excessive and precocious glutamate release in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology 46:782–792. https://doi.org/10.1016/j.neuropharm.2003.11.025
Raiteri L, Zappettini S, Stigliani S, Paluzzi S, Raiteri M, Bonanno G (2005) Glutamate release induced by activation of Glycine and GABA transporters in spinal cord is enhanced in a mouse model of amyotrophic lateral sclerosis. Neurotoxicology 26:883–892. https://doi.org/10.1016/j.neuro.2005.01.015
Giribaldi F, Milanese M, Bonifacino T, Anna Rossi PI, di Prisco S, Pittaluga A, Tacchetti C, Puliti A et al (2013) Group I metabotropic glutamate autoreceptors induce abnormal glutamate exocytosis in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology 66:253–263. https://doi.org/10.1016/j.neuropharm.2012.05.018
Milanese M, Zappettini S, Onofri F, Musazzi L, Tardito D, Bonifacino T, Messa M, Racagni G et al (2011) Abnormal exocytotic release of glutamate in a mouse model of amyotrophic lateral sclerosis. J Neurochem 116:1028–1042. https://doi.org/10.1111/j.1471-4159.2010.07155.x
Bonifacino T, Musazzi L, Milanese M, Seguini M, Marte A, Gallia E, Cattaneo L, Onofri F et al (2016) Altered mechanisms underlying the abnormal glutamate release in amyotrophic lateral sclerosis at a pre-symptomatic stage of the disease. Neurobiol Dis 95:122–133. https://doi.org/10.1016/j.nbd.2016.07.011
Milanese M, Bonifacino T, Fedele E, Rebosio C, Cattaneo L, Benfenati F, Usai C, Bonanno G (2015) Exocytosis regulates trafficking of GABA and glycine heterotransporters in spinal cord glutamatergic synapses: a mechanism for the excessive heterotransporter-induced release of glutamate in experimental amyotrophic lateral sclerosis. Neurobiol Dis 74:314–324. https://doi.org/10.1016/j.nbd.2014.12.004
Milanese M, Zappettini S, Jacchetti E, Bonifacino T, Cervetto C, Usai C, Bonanno G (2010) In vitro activation of GAT1 transporters expressed in spinal cord gliosomes stimulates glutamate release that is abnormally elevated in the SOD1/G93A(+) mouse model of amyotrophic lateral sclerosis. J Neurochem 113:489–501. https://doi.org/10.1111/j.1471-4159.2010.06628.x
Whittaker VP (1993) Thirty years of synaptosome research. J Neurocytol 22:735–742
Raiteri M (1983) Synaptosomes as a tool in the development of new neuroactive drugs. Rev Pure Appl Pharmacol Sci 4:65–109
Nakamura Y, Iga K, Shibata T, Shudo M, Kataoka K (1993) Glial plasmalemmal vesicles: a subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia 9:48–56. https://doi.org/10.1002/glia.440090107
Stigliani S, Zappettini S, Raiteri L, Passalacqua M, Melloni E, Venturi C, Tacchetti C, Diaspro A et al (2006) Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J Neurochem 96:656–668. https://doi.org/10.1111/j.1471-4159.2005.03631.x
Carney KE, Milanese M, Van Nierop P et al (2014) Proteomic analysis of gliosomes from mouse brain: identification and investigation of glial membrane proteins. J Proteome Res 13:5918–5927. https://doi.org/10.1021/pr500829z
Gurney ME, Pu H, Chiu AY, Dal Canto M, Polchow C, Alexander D, Caliendo J, Hentati A et al (1994) Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264:1772–1775
Bonifacino T, Cattaneo L, Gallia E, Puliti A, Melone M, Provenzano F, Bossi S, Musante I et al (2017) In-vivo effects of knocking-down metabotropic glutamate receptor 5 in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuropharmacology 123:433–445. https://doi.org/10.1016/j.neuropharm.2017.06.020
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cappelli E, Cuccarolo P, Stroppiana G, et al. (2017) Defects in mitochondrial energetic function compels Fanconi Anemia cells to glycolytic metabolism. BBA - Mol Basis Dis. doi: https://doi.org/10.1016/j.bbadis.2017.03.008
Ravera S, Panfoli I, Calzia D, Aluigi MG, Bianchini P, Diaspro A, Mancardi G, Morelli A (2009) Evidence for aerobic ATP synthesis in isolated myelin vesicles. Int J Biochem Cell Biol 41:1581–1591
Ravera S, Panfoli I, Aluigi MG, Calzia D, Morelli A (2011) Characterization of myelin sheath F(o)F(1)-ATP synthase and its regulation by IF(1). Cell Biochem Biophys 59:63–70
Ravera S, Bartolucci M, Cuccarolo P, Litamè E, Illarcio M, Calzia D, Degan P, Morelli A et al (2015) Oxidative stress in myelin sheath: the other face of the extramitochondrial oxidative phosphorylation ability. Free Radic Res 49:1–36. https://doi.org/10.3109/10715762.2015.1050962
Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT (2005) Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci 25:164–172. https://doi.org/10.1523/JNEUROSCI.3829-04.2005
Boyer PD (2002) A research journey with ATP synthase. J Biol Chem 277:39045–39061
Collard J-F, Côté F, Julien J-P (1995) Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature 375:61–64. https://doi.org/10.1038/375061a0
Williamson TL, Cleveland DW (1999) Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci 2:50–56. https://doi.org/10.1038/4553
Pfanner N, Meijer M (1997) Mitochondrial biogenesis: The Tom and Tim machine. Curr Biol 7:R100–R103. https://doi.org/10.1016/S0960-9822(06)00048-0
Capaldi RA, Malatesta F, Darley-Usmar VM (1983) Structure of cytochrome c oxidase. Biochim Biophys Acta - Rev Bioenerg 726:135–148. https://doi.org/10.1016/0304-4173(83)90003-4
Cadenas E, Davies KJA (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230. https://doi.org/10.1016/S0891-5849(00)00317-8
Sasaki S, Iwata M (2007) Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 66:10–16. https://doi.org/10.1097/nen.0b013e31802c396b
Ghijsen WEJM, Leenders AGM, Lopes da Silva FH (2003) Regulation of vesicle traffic and neurotransmitter release in isolated nerve terminals. Neurochem Res 28:1443–1452
Ferraiuolo L, Higginbottom A, Heath PR, Barber S, Greenald D, Kirby J, Shaw PJ (2011) Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 134:2627–2641. https://doi.org/10.1093/brain/awr193
Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, Bonanno G (2007) Adult astroglia is competent for Na + /Ca 2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem 103:1196–1207. https://doi.org/10.1111/j.1471-4159.2007.04826.x
Tsujimoto Y, Nakagawa T, Shimizu S (2006) Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta Bioenerg 1757:1297–1300. https://doi.org/10.1016/j.bbabio.2006.03.017
Quintanilla RA, Jin YN, von Bernhardi R, Johnson GVW (2013) Mitochondrial permeability transition pore induces mitochondria injury in Huntington disease. Mol Neurodegener 8:45. https://doi.org/10.1186/1750-1326-8-45
Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128:1271–1281. https://doi.org/10.1104/pp.010999
Dai D-F, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS (2014) Mitochondrial oxidative stress in aging and healthspan. Longev Heal 3:6. https://doi.org/10.1186/2046-2395-3-6
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031. https://doi.org/10.1074/jbc.M304854200
Lenaz G (2001) The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52:159–164. https://doi.org/10.1080/15216540152845957
Fang C, Bourdette D, Banker G (2012) Oxidative stress inhibits axonal transport: implications for neurodegenerative diseases. Mol Neurodegener 7:29. https://doi.org/10.1186/1750-1326-7-29
Boillée S, Vande Velde C, Cleveland DWW (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52:39–59. https://doi.org/10.1016/j.neuron.2006.09.018
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H et al (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11:251–253. https://doi.org/10.1038/nn2047
Acknowledgements
The authors are indebted to Mr. Giuseppe Marazzotta for his help in maintaining the SOD1G93Aand the wtSOD1 mouse colonies. The authors gratefully acknowledge the undergraduate students Cesare Cecchini, Mirna Nitro, and Roncallo Roberta, for their helpful technical support.
Funding
This work was supported by research grants from the Italian Ministry of University (PRIN Project No. 2016058401 to GB and SIR Project No. RBSI14B1Z to M.M.) and from the Motor Neuron Disease Association (Project No. April16/848-791) to GB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ravera, S., Bonifacino, T., Bartolucci, M. et al. Characterization of the Mitochondrial Aerobic Metabolism in the Pre- and Perisynaptic Districts of the SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Mol Neurobiol 55, 9220–9233 (2018). https://doi.org/10.1007/s12035-018-1059-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1059-z