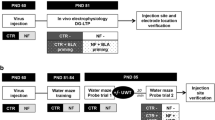
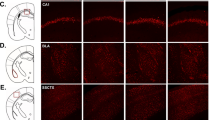
Abstract
Repeated stress causes cognitive decline and decreases the expression of glial fibrillary acidic protein (GFAP)+ astroglial cells in the prefrontal cortex (PFC). The stress-induced alterations in astroglial density and morphology might significantly contribute to cognitive impairments. Apart from PFC, a key region involved in modulation of repercussions of stress is basolateral amygdala (BLA), which undergoes hypertrophy following chronic immobilization stress (CIS) and has intense reciprocal connections to the PFC. Interestingly, inactivation of BLA precludes stress-induced learning deficits. However, the modulatory role of BLA on CIS-induced alterations in GFAP+ astroglial density and associated learning deficits are presently unknown. Accordingly, we present two sets of experiments evaluating the effects of BLA inactivation either permanently or temporarily on CIS-induced changes in learning and astroglial expression in the PFC. CIS causes impairment in novel object recognition memory and astroglial loss in the PFC. In experiment I, we permanently inactivated the BLA by ibotenate lesion prior to CIS and observed a significant improvement in learning. Surprisingly, BLA lesion also prevented the stress-induced astroglial loss in the PFC. Furthermore, in the experiment II, we analyzed whether the effects of permanent inactivation could be mirrored by the temporary blockage of BLA specifically during stress. Interestingly, temporary inactivation of BLA mimics the effects of lesion. There was a notable prevention of learning impairment and astroglial loss in the PFC following BLA inactivation during stress. The present study emphasizes that stress-induced astroglial loss might contribute to cognitive deficits and modulation of BLA activity might be a viable strategy for management of stress-related PFC dysfunctions.









Similar content being viewed by others
Abbreviations
- BLA:
-
Basolateral amygdala
- PFC:
-
Prefrontal cortex
- PrL:
-
Prelimbic cortex
- ACC:
-
Anterior cingulate cortex
- GFAP:
-
Glial fibrillary acidic protein
- CIS:
-
Chronic immobilization stress
- NOR:
-
Novel object recognition
- HPA axis:
-
Hypothalamic pituitary adrenal axis
- NMDA:
-
N-methyl-d-aspartate
References
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012) Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73(5):962–977
Ramkumar K, Srikumar BN, Venkatasubramanian D, Siva R, Shankaranarayana Rao BS, Raju TR (2012) Reversal of stress-induced dendritic atrophy in the prefrontal cortex by intracranial self-stimulation. J Neural Transm (Vienna) 119(5):533–543
Pawlak R, Shankaranarayana Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S (2005) Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci U S A 102(50):18201–18206
Shilpa BM, Bhagya V, Harish G, Srinivas Bharath MM, Shankaranarayana Rao BS (2017) Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Prog Neuro-Psychopharmacol Biol Psychiatry 76:88–100
Veena J, Srikumar BN, Raju TR, Shankaranarayana Rao BS (2009) Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett 455(3):178–182
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93(3):358–364
Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S (2003) Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci 6(2):168–174
Wei J, Zhong P, Qin L, Tan T, Yan Z (2017) Chemicogenetic restoration of the prefrontal cortex to amygdala pathway ameliorates stress-induced deficits. Cereb Cortex. https://doi.org/10.1093/cercor/bhx104
Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31(8):1616–1626
Banasr M, Duman RS (2008) Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry 64(10):863–870
Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR (2013) Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol 126(1):75–91
Newman EA (2003) New roles for astrocytes: Regulation of synaptic transmission. Trends Neurosci 26(10):536–542
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32(8):421–431
Kant D, Tripathi SS, Qureshi MF, Tripathi S 2nd, Pandey S, Singh G, Kumar T, Mir FA et al (2014) The effect of glial glutamine synthetase inhibition on recognition and temporal memories in the rat. Neurosci Lett 560:98–102
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144(5):810–823
Gibbs ME, Hertz L, Ng KT (2004) Inhibition of short-term memory formation in the chick by blockade of extracellular glutamate uptake. Neurobiol Learn Mem 81(2):115–119
Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, Huitron-Resendiz S, Pina-Crespo JC et al (2014) Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A 111(32):E3343–E3352
Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J et al (2015) Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci 18(3):423–434
Roozendaal B, Portillo-Marquez G, McGaugh JL (1996) Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav Neurosci 110(5):1074–1083
Roozendaal B, McReynolds JR, McGaugh JL (2004) The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci 24(6):1385–1392
Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22(15):6810–6818
Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL et al (2008) Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 507(1):1141–1150
Wellman CL (2001) Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol 49(3):245–253
Amaral DG, Price JL (1984) Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230(4):465–496
Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212(2):149–179
Krettek JE, Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171(2):157–191
Little JP, Carter AG (2013) Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci 33(39):15333–15342
Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK (2009) Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci 29(45):14299–14308
Maroun M (2006) Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur J Neurosci 24(10):2917–2922
Maroun M, Richter-Levin G (2003) Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci 23(11):4406–4409
Roozendaal B, Sapolsky RM, McGaugh JL (1998) Basolateral amygdala lesions block the disruptive effects of long-term adrenalectomy on spatial memory. Neuroscience 84(2):453–465
Roozendaal B, Nguyen BT, Power AE, McGaugh JL (1999) Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci U S A 96(20):11642–11647
Rei D, Mason X, Seo J, Graff J, Rudenko A, Wang J, Rueda R, Siegert S et al (2015) Basolateral amygdala bidirectionally modulates stress-induced hippocampal learning and memory deficits through a p25/Cdk5-dependent pathway. Proc Natl Acad Sci U S A 112(23):7291–7296
Jeon B, Hwang YK, Lee SY, Kim D, Chung C, Han JS (2012) The role of basolateral amygdala in the regulation of stress-induced phosphorylated extracellular signal-regulated kinase expression in the hippocampus. Neuroscience 224:191–201
Kim JJ, Lee HJ, Han JS, Packard MG (2001) Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci 21(14):5222–5228
Kim JJ, Koo JW, Lee HJ, Han JS (2005) Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci 25(6):1532–1539
Tripathi SJ, Chakraborty S, Srikumar BN, Raju TR, Shankaranarayana Rao BS (2017) Inactivation of basolateral amygdala prevents chronic immobilization stress-induced memory impairment and associated changes in corticosterone levels. Neurobiol Learn Mem 142(Pt B):218–229
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates. Academic Press, Amsterdam
Tripathi SJ, Chakraborty S, Srikumar BN, Raju TR, Shankaranarayana Rao BS (2017) Prevention of chronic immobilization stress-induced enhanced expression of glucocorticoid receptors in the prefrontal cortex by inactivation of basolateral amygdala. J Chem Neuroanat. https://doi.org/10.1016/j.jchemneu.2017.12.006
Nibuya M, Takahashi M, Russell DS, Duman RS (1999) Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett 267(2):81–84
Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL (2006) Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A 103(17):6741–6746
de Lima MN, Laranja DC, Bromberg E, Roesler R, Schroder N (2005) Pre- or post-training administration of the NMDA receptor blocker MK-801 impairs object recognition memory in rats. Behav Brain Res 156(1):139–143
Karasawa J, Hashimoto K, Chaki S (2008) D-serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res 186(1):78–83
Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Shankaranarayana Rao BS (2009) Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res 87(4):831–843
Veena J, Srikumar BN, Mahati K, Raju TR, Shankaranarayana Rao BS (2011) Oxotremorine treatment restores hippocampal neurogenesis and ameliorates depression-like behaviour in chronically stressed rats. Psychopharmacology 217(2):239–253
Abhijit S, Tripathi SJ, Bhagya V, Shankaranarayana Rao BS, Subramanyam MV, Asha Devi S (2018) Antioxidant action of grape seed polyphenols and aerobic exercise in improving neuronal number in the hippocampus is associated with decrease in lipid peroxidation and hydrogen peroxide in adult and middle-aged rats. Exp Gerontol 101:101–112
Vrinda M, Sasidharan A, Aparna S, Srikumar BN, Kutty BM, Shankaranarayana Rao BS (2017) Enriched environment attenuates behavioral seizures and depression in chronic temporal lobe epilepsy. Epilepsia 58(7):1148–1158
Shankaranarayana Rao BS, Govindaiah, Laxmi TR, Meti BL, Raju TR (2001) Subicular lesions cause dendritic atrophy in CA1 and CA3 pyramidal neurons of the rat hippocampus. Neuroscience 102(2):319–327
Shankaranarayana Rao BS, Desiraju T, Raju TR (1993) Neuronal plasticity induced by self-stimulation rewarding experience in rats—a study on alteration in dendritic branching in pyramidal neurons of hippocampus and motor cortex. Brain Res 627(2):216–224
West MJ (1999) Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci 22(2):51–61
Ennaceur A, Neave N, Aggleton JP (1997) Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113(3):509–519
Gaskin S, Tardif M, Cole E, Piterkin P, Kayello L, Mumby DG (2010) Object familiarization and novel-object preference in rats. Behav Process 83(1):61–71
Baxter MG (2010) "I've seen it all before": explaining age-related impairments in object recognition. Theoretical comment on Burke et al. (2010). Behav Neurosci 124(5):706–709
Nishimura KJ, Ortiz JB, Conrad CD (2017) Antagonizing the GABAA receptor during behavioral training improves spatial memory at different doses in control and chronically stressed rats. Neurobiol Learn Mem 145:114–118
Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N (2007) The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 27(11):2781–2787
Srikumar BN, Raju TR, Shankaranarayana Rao BS (2006) The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience 143(3):679–688
McLaughlin KJ, Gomez JL, Baran SE, Conrad CD (2007) The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res 1161:56–64
Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ (1995) Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci 15(1 Pt 1):61–69
Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O'Mara SM (2016) Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep 6:29127
Sunanda, Shankaranarayana Rao BS, Raju TR (2000) Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Curr Sci 79(11):1581
Shankaranarayana Rao BS, Madhavi R, Sunanda RTR (2001) Complete reversal of dendritic atrophy in CA3 neurons of the hippocampus by rehabilitation in restraint stressed rats. Curr Sci 80:653–659
Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR (2013) Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 23(8):1784–1797
Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N (2005) Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 25(34):7792–7800
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005) Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A 102(26):9371–9376
Govindarajan A, Shankaranarayana Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S (2006) Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A 103(35):13208–13213
Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM (2013) BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79(4):658–664
Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM (2016) Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321:197–209
Parent MB, Avila E, McGaugh JL (1995) Footshock facilitates the expression of aversively motivated memory in rats given post-training amygdala basolateral complex lesions. Brain Res 676(2):235–244
Maren S, Aharonov G, Fanselow MS (1996) Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci 110(4):718–726
Steiner J, Bernstein HG, Bielau H, Berndt A, Brisch R, Mawrin C, Keilhoff G, Bogerts B (2007) Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci 8:2
Steiner J, Bernstein HG, Bogerts B, Gos T, Richter-Landsberg C, Wunderlich MT, Keilhoff G (2008) S100B is expressed in, and released from, OLN-93 oligodendrocytes: influence of serum and glucose deprivation. Neuroscience 154(2):496–503
Si X, Miguel-Hidalgo JJ, O'Dwyer G, Stockmeier CA, Rajkowska G (2004) Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology 29(11):2088–2096
Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G (2000) Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry 48(8):861–873
Rajkowska G, Miguel-Hidalgo JJ (2007) Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets 6(3):219–233
Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS (2004) Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry 161(4):637–645
Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J (2008) A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 106(1–2):1–27
Lima A, Sardinha VM, Oliveira AF, Reis M, Mota C, Silva MA, Marques F, Cerqueira JJ et al (2014) Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Mol Psychiatry 19(7):834–841
Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G (2010) Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15(5):501–511
Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E et al (2017) Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22(1):120–126
Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA et al (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16(3):675–686
Rappeneau V, Blaker A, Petro JR, Yamamoto BK, Shimamoto A (2016) Disruption of the glutamate-glutamine cycle involving astrocytes in an animal model of depression for males and females. Front Behav Neurosci 10:231
Mookherjee P, Green PS, Watson GS, Marques MA, Tanaka K, Meeker KD, Meabon JS, Li N et al (2011) GLT-1 loss accelerates cognitive deficit onset in an Alzheimer's disease animal model. J Alzheimers Dis 26(3):447–455
Li SX, Han Y, Xu LZ, Yuan K, Zhang RX, Sun CY, Xu DF, Yuan M et al (2018) Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry 23(3):597–608
Wang J, Tu J, Cao B, Mu L, Yang X, Cong M, Ramkrishnan AS, Chan RHM et al (2017) Astrocytic l-lactate signaling facilitates amygdala-anterior cingulate cortex synchrony and decision making in rats. Cell Rep 21(9):2407–2418
Popoli M, Yan Z, McEwen BS, Sanacora G (2011) The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13(1):22–37
Sheline YI (2000) 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry 48(8):791–800
Acknowledgements
Sunil Jamuna Tripathi was supported by a Senior Research Fellowship (File No. 09/490(0095)/2014-EMR-I) from the Council of Scientific and Industrial Research (CSIR), New Delhi, India. Suwarna Chakraborty was supported by a research fellowship (NIMH: A&E/C:PhD(NP):2013-14: SC) from the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India. We acknowledge financial support from the Department of Biotechnology (DBT), Science and Engineering Research Board, Department of Science & Technology, Government of India (SERB-DST), New Delhi and National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India.
Contributions
S.J.T., T.R.R., and B.S.S.R. conceptualized and designed the experiments; S.J.T. and S.C. performed the experiments and analyzed the data; B.N.S, T.R.R., and B.S.S.R. contributed to reagents/materials/analysis tools; and S.J.T., S.C., B.N.S, T.R.R., and B.S.S.R. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Electronic Supplementary Material
ESM 1
(PDF 499 kb)
Rights and permissions
About this article
Cite this article
Tripathi, S.J., Chakraborty, S., Srikumar, B.N. et al. Inactivation of Basolateral Amygdala Prevents Stress-Induced Astroglial Loss in the Prefrontal Cortex. Mol Neurobiol 56, 350–366 (2019). https://doi.org/10.1007/s12035-018-1057-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1057-1