Abstract
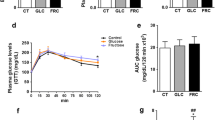
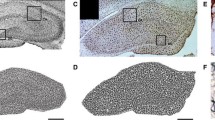
There has been a progressive increase in the incidence of fructose-induced metabolic disorders, such as metabolic syndrome (MetS). Moreover, novel evidence reported negative effects of high-fructose diets in brain function. This study was designed to evaluate for the first time the effects of long-term fructose consumption (LT-FC) on the normal ageing process in a long-lived animal model rodent, Octodon degus or degu. Moreover, we could replicate human sugar consumption behaviour over time, leading us to understand then the possible mechanisms by which this MetS-like condition could affect cognitive abilities. Our results support that 28 months (from pup to adulthood) of a 15% solution of fructose induced clinical conditions similar to MetS which includes an insulin-resistance scenario together with elevated basal metabolic rate and non-alcoholic fatty liver disease. Additionally, we extended our analysis to evaluate the impact of this MetS-like condition on the functional and cognitive brain processes. Behavioural test suggests that fructose-induced MetS-like condition impair hippocampal-dependent and independent memory performance. Moreover, we also reported several neuropathological events as impaired hippocampal redox balance, together with synaptic protein loss. These changes might be responsible for the alterations in synaptic plasticity and transmitter release observed in these cognitively impaired animals. Our results indicate that LT-FC induced several facets of MetS that eventually could trigger brain disorders, in particular, synaptic dysfunction and reduced cognition.









Similar content being viewed by others
References
Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ (2008) Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Obes Surg 18(8):1028–1034
Miller A, Adeli K (2008) Dietary fructose and the metabolic syndrome. Curr Opin Gastroenterol 224(2):204–209
Jürgens H, Haass W, Castañeda TR, Schürmann A, Koebnick C, Dombrowski F, Otto B, Nawrocki AR et al (2005) Consuming fructose-sweetened beverages increases body adiposity in mice. Obesity 13(7):1146–1156
Rizkalla SW (2010) Health implications of fructose consumption: a review of recent data. Nutr Metab 7(1):82
Tappy L, Lê K-A (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90(1):23–46
Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K (2010) Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 299(5):E685–E694
Malik VS, Hu FB (2012) Sweeteners and risk of obesity and type 2 diabetes: the role of sugar-sweetened beverages. Curr Diab Rep 12(2):195–203
Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T et al (2005) The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 143(10):722–728
Ríos JA, Cisternas P, Arrese M, Barja S, Inestrosa NC (2014) Is Alzheimer’s disease related to metabolic syndrome? A Wnt signaling conundrum. Prog Neurobiol 121:125–146
Suzanne M, Tong M (2014) Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol 88(4):548–559
Gómez-Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9(7):568–578
Agrawal R, Gomez-Pinilla F (2012) ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol 590(10):2485–2499
Ross AP, Bartness TJ, Mielke JG, Parent MB (2009) A high fructose diet impairs spatial memory in male rats. Neurobiol Learn Mem 92(3):410–416
Cisternas P, Salazar P, Serrano FG, Montecinos-Oliva C, Arredondo SB, Varela-Nallar L, Barja S, Vio CP et al (2015) Fructose consumption reduces hippocampal synaptic plasticity underlying cognitive performance. Biochim Biophys Acta BBA-Mol Basis Dis 1852(11):2379–2390
Agrawal R, Noble E, Vergnes L, Ying Z, Reue K, Gomez-Pinilla F (2016) Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. J Cereb Blood Flow Metab 36(5):941–953
Levi B, Werman MJ (1998) Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J Nutr 128(9):1442–1449
Gomez-Pinilla F, Tyagi E (2013) Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care 16(6):726–733
Tillman EJ, Morgan DA, Rahmouni K, Swoap SJ (2014) Three months of high-fructose feeding fails to induce excessive weight gain or leptin resistance in mice. PLoS One 9(9):e107206
Livesey G, Taylor R (2008) Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 88(5):1419–1437
White JS (2013) Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr Int Rev J 4(2):246–256
Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang D-H, Sánchez-Lozada LG (2007) Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86(4):899–906
Havel PJ (2005) Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 63(5):133–157
Sánchez-Lozada LG, Tapia E, Jiménez A, Bautista P, Cristóbal M, Nepomuceno T, Soto V, Avila-Casado C et al (2007) Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol-Ren Physiol 292(1):F423–F429
Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ (2011) Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci 4(4):243–252
Edwards MS (2009) Nutrition and behavior of degus (Octodon degus). Veterinary Clin North Am Exot Anim Pract 12(2):237–253
Homan R, Hanselman JC, Bak-Mueller S, Washburn M, Lester P, Jensen HE, Pinkosky SL, Castle C et al (2010) Atherosclerosis in Octodon degus (degu) as a model for human disease. Atherosclerosis 212(1):48–54
Brown C, Donnelly TM (2001) Cataracts and reduced fertility in degus (Octodon degus). Contracts secondary to spontaneous diabetes mellitus. Lab Anim 30(6):25
Castro-Fuentes R, Socas-Pérez R (2013) Octodon degus: a strong attractor for Alzheimer research. Basic Clin Neurosci 4(1):91–96
Opazo JC, Soto-Gamboa M, Bozinovic F (2004) Blood glucose concentration in caviomorph rodents. Comp Biochem Physiol A Mol Integr Physiol 137(1):57–64
Ardiles ÁO, Tapia-Rojas CC, Mandal M, Alexandre F, Kirkwood A, Inestrosa NC, Palacios AG (2012) Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer’s disease. Proc Natl Acad Sci 109(34):13835–13840
Rivera DS, Inestrosa NC, Bozinovic F (2016) On cognitive ecology and the environmental factors that promote Alzheimer disease: lessons from Octodon degus (Rodentia: Octodontidae). Biol Res 49(1):10
Inestrosa NC, Ríos JA, Cisternas P, Tapia-Rojas C, Rivera DS, Braidy N, Zolezzi JM, Godoy JA et al (2015) Age progression of neuropathological markers in the brain of the Chilean rodent Octodon degus, a natural model of Alzheimer’s disease. Brain Pathol 25(6):679–691
Veloso C, Bozinovic F (1993) Dietary and digestive constraints on basal energy metabolism in a small herbivorous rodent. Ecology 74(7):2003–2010
Lott JA, Turner K (1975) Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin Chem 21(12):1754–1760
Johnson RN, Metcalf PA, Baker JR (1983) Fructosamine: a new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin Chim Acta 127(1):87–95
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Group NDD (1979) Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 28(12):1039–1057
Bozinovic F, Bacigalupe LD, Vasquez RA, Visser GH, Veloso C, Kenagy GJ (2004) Cost of living in free-ranging degus (Octodon degus): seasonal dynamics of energy expenditure. Comp Biochem Physiol A Mol Integr Physiol 137(3):597–604
Bozinovic F, Rojas JM, Broitman BR, Vásquez RA (2009) Basal metabolism is correlated with habitat productivity among populations of degus (Octodon degus). Comp Biochem Physiol A Mol Integr Physiol 152(4):560–564
Withers PC (1977) Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. J Appl Physiol 42(1):120–123
Takahashi Y, Fukusato T (2014) Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol WJG 20(42):15539–15548
Cabrera D, Ruiz A, Cabello-Verrugio C, Brandan E, Estrada L, Pizarro M, Solis N, Torres J et al (2016) Diet-induced nonalcoholic fatty liver disease is associated with sarcopenia and decreased serum insulin-like growth factor-1. Dig Dis Sci 61(11):3190–3198
Rivera DS, Lindsay C, Codocedo JF, Morel I, Pinto C, Cisternas P, Bozinovic F, Inestrosa NC (2016) Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol Aging 46:204–220
Kumazawa-Manita N, Hama H, Miyawaki A, Iriki A (2013) Tool use specific adult neurogenesis and synaptogenesis in rodent (Octodon degus) hippocampus. PLoS One 8(3):e58649
Barnes CA (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93(1):74–104
Serrano FG, Tapia-Rojas C, Carvajal FJ, Hancke J, Cerpa W, Inestrosa NC (2014) Andrographolide reduces cognitive impairment in young and mature AβPPswe/PS-1 mice. Mol Neurodegener 9(1):61
Aydin S, Aksoy A, Aydin S, Kalayci M, Yilmaz M, Kuloglu T, Citil C, Catak Z (2014) Today’s and yesterday’s of pathophysiology: biochemistry of metabolic syndrome and animal models. Nutrition 30(1):1–9
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E et al (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37(4):917–923
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P (2015) Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 47(3):181–190
Singh S, Nath P, Parida P, Narayan J, Padhi P, Singh A, Pati G, Agrawal O et al (2015) NAFLD alone is a better predictor than MS (ATP-III) criteria for insulin resistance. J Clin Exp Hepatol 5:S21
Arrese M (2010) Nonalcoholic fatty liver disease: liver disease: an overlooked complication of diabetes mellitus. Nat Rev Endocrinol 6(12):660–661
Targher G (2007) Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med 24(1):1–6
Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA (2005) Fructose-induced fatty liver disease. Hypertension 45(5):1012–1018
Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C (2007) Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem 18(3):184–195
Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ (1989) Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr 49(5):832–839
Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL et al (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119(5):1322–1334
Hwang I-S, Ho H, Hoffman BB, Reaven GM (1987) Fructose-induced insulin resistance and hypertension in rats. Hypertension 10(5):512–516
Kamide K, Rakugi H, Higaki J, Okamura A, Nagai M, Moriguchi K, Ohishi M, Satoh N et al (2002) The renin-angiotensin and adrenergic nervous system in cardiac hypertrophy in fructose-fed rats. Am J Hypertens 15(1):66–71
Axelsen LN, Lademann JB, Petersen JS, Holstein-Rathlou N-H, Ploug T, Prats C, Pedersen HD, Kjølbye AL (2010) Cardiac and metabolic changes in long-term high fructose-fat fed rats with severe obesity and extensive intramyocardial lipid accumulation. Am J Physiol-Regul Integr Comp Physiol 298(6):R1560–R1570
Ha V, Sievenpiper JL, de Souza RJ, Chiavaroli L, Wang DD, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Dibuono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ (2012) Effect of fructose on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Hypertension HYPERTENSIONAHA–111.
Farah D, Nunes J, Sartori M, da Silva Dias D, Sirvente R, Silva MB, Fiorino P, Morris M et al (2016) Exercise training prevents cardiovascular derangements induced by fructose overload in developing rats. PLoS One 11(12):e0167291
Balakumar M, Raji L, Prabhu D, Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M (2016) High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol Cell Biochem 423(1–2):93–104
Lim JS, Mietus-Snyder M, Valente A, Schwarz J-M, Lustig RH (2010) The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 7(5):251–264
Abdelmalek MF (2010) Nonalcoholic steatohepatitis clinical research network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51:1961–1971
Sellmann C, Priebs J, Landmann M, Degen C, Engstler AJ, Jin CJ, Gärttner S, Spruss A et al (2015) Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J Nutr Biochem 26(11):1183–1192
Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KH, Schwarze M, Manns MP (1999) Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr 69(6):1194–1201
Tarantino G, Marra M, Contaldo F, Pasanisi F (2008) Basal metabolic rate in morbidly obese patients with non-alcoholic fatty liver disease. Clin Invest Med 31(1):24–29
Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G et al (2005) Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 1(2):107–119
Woods SC, Seeley RJ, Porte D, Schwartz MW (1998) Signals that regulate food intake and energy homeostasis. Science 280(5368):1378–1383
Haleem DJ, Haider S, Perveen T, Inam Q-U-A, Kidwai IM, Haleem MA (2000) Hyperphagia and decreases of brain serotonin in rats fed freely on sugar rich diet for three weeks. Nutr Neurosci 3(6):399–405
Sohal RS (2002) Role of oxidative stress and protein oxidation in the aging process 1, 2. Free Radic Biol Med 33(1):37–44
Burton T, Killen SS, Armstrong JD, Metcalfe NB (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc R Soc Lond B Biol Sci 278(1724):3465–3473
Mastrocola R, Nigro D, Cento AS, Chiazza F, Collino M, Aragno M (2016) High-fructose intake as risk factor for neurodegeneration: key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol Dis 89:65–75
Yan L-J, Levine RL, Sohal RS (1997) Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci 94(21):11168–11172
Sohal RS, Ku H-H, Agarwal S, Forster MJ, Lal H (1994) Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev 74(1):121–133
Lopes A, Vilela TC, Taschetto L, Vuolo F, Petronilho F, Dal-Pizzol F, Streck EL, Ferreira GC et al (2014) Evaluation of the effects of fructose on oxidative stress and inflammatory parameters in rat brain. Mol Neurobiol 50(3):1124–1130
Castro MC, Massa ML, Arbeláez LG, Schinella G, Gagliardino JJ, Francini F (2015) Fructose-induced inflammation, insulin resistance and oxidative stress: a liver pathological triad effectively disrupted by lipoic acid. Life Sci 137:1–6
Calvo-Ochoa E, Hernández-Ortega K, Ferrera P, Morimoto S, Arias C (2014) Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. J Cereb Blood Flow Metab 34(6):1001–1008
Hudoyo AW, Hirase T, Tandelillin A, Honda M, Shirai M, Cheng J, Morisaki H, Morisaki T (2017) Role of AMPD2 in impaired glucose tolerance induced by high fructose diet. Mol Genet Metab Rep 13:23–29
Li P, Koike T, Qin B, Kubota M, Kawata Y, Jia YJ, Oshida Y (2008) A high-fructose diet impairs Akt and PKCζ phosphorylation and GLUT4 translocation in rat skeletal muscle. Horm Metab Res 40(08):528–532
Pham N, Dhar A, Khalaj S, Desai K, Taghibiglou C (2014) Down regulation of brain cellular prion protein in an animal model of insulin resistance: possible implication in increased prevalence of stroke in pre-diabetics/diabetics. Biochem Biophys Res Commun 448(2):151–156
Cao D, Lu H, Lewis TL, Li L (2007) Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem 282(50):36275–36282
Kogan JH, Frankland PW, Silva AJ (2000) Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10(1):47–56
Rosenfeld CS, Ferguson SA (2014) Barnes maze testing strategies with small and large rodent models. J Vis Exp JoVE 84:e51194
Inam Q-A, Ikram H, Shireen E, Haleem DJ (2016) Effects of sugar rich diet on brain serotonin, hyperphagia and anxiety in animal model of both genders. Pak J Pharm Sci 29(3):757–763
Yoshimi N, Futamura T, Hashimoto K (2015) Improvement of dizocilpine-induced social recognition deficits in mice by brexpiprazole, a novel serotonin–dopamine activity modulator. Eur Neuropsychopharmacol 25(3):356–364
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110
Jarrard LE (1993) On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol 60(1):9–26
Fusco S, Pani G (2013) Brain response to calorie restriction. Cell Mol Life Sci 70(17):3157–3170
Harris KM, Weinberg RJ (2012) Ultrastructure of synapses in the mammalian brain. Cold Spring Harb Perspect Biol 4(5):a005587
Lüscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4(6):a005710
Hessler NA, Shirke AM, Malinow R (1993) The probability of transmitter release at a mammalian central synapse. Nature 366(6455):569–572
Murthy VN, Sejnowski TJ, Stevens CF (1997) Heterogeneous release properties of visualized individual hippocampal synapses. Neuron 18(4):599–612
Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA et al (2015) PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc Natl Acad Sci 112(50):E6983–E6992
Nagura H, Ishikawa Y, Kobayashi K, Takao K, Tanaka T, Nishikawa K, Tamura H, Shiosaka S et al (2012) Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice. Mol Brain 5(1):43
Acknowledgements
This work was supported by a postdoctoral grant from Fondo Nacional de Desarollo Científico y Tecnológico (FONDECYT) N° 3140395 to DSR. PC was supported by FONDECYT N° 11160651. MAA was supported by FONDECYT N°1150327. DC was supported by FONDECYT N° 11171001. NCI was supported by FONDECYT N° 1160724 and grants from the Basal Centre of Excellence in Science and Technology (CONICYT-PFB12/2007) and AFB 170005. In addition, a grant from CAPES-CONICYT FB 0002-2014 (Line 3) was awarded to FB. We thank G. Cavieres for assistance with Basal metabolic rate records, C. Céspedes for assistance with blood pressure measurements and J. Rios for assistance with liver histopathology analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Housing, treatment and euthanasia of animals met the guidelines set by the National Institutes of Health (NIH, Baltimore, MD, USA). All experimental procedures involving animals were approved by the Bioethical and Biosafety Committee of the Faculty of Biological Sciences of the Pontificia Universidad Católica de Chile (CBB-121-2013). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rivera, D.S., Lindsay, C.B., Codocedo, J.F. et al. Long-Term, Fructose-Induced Metabolic Syndrome-Like Condition Is Associated with Higher Metabolism, Reduced Synaptic Plasticity and Cognitive Impairment in Octodon degus. Mol Neurobiol 55, 9169–9187 (2018). https://doi.org/10.1007/s12035-018-0969-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0969-0