Abstract
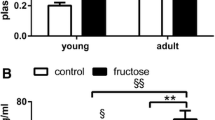
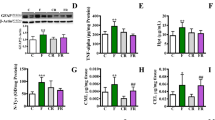
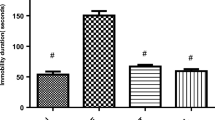
Although numerous studies have demonstrated the harmful effect of excessive fructose consumption at the systemic level, there is little information on its effects in the central nervous system. The purpose of the present work was to study the cellular alterations related to oxidative stress and protein quality control systems induced by a high-fructose diet in the brain of Syrian hamsters and their possible attenuation by exogenous melatonin. High-fructose intake induced type II diabetes together with oxidative damage, led to alterations of the unfolded protein response by activating the eIF2α branch, and impaired the macroautophagic machinery in the brain, favoring the accumulation of aggregates labeled for selective degradation and neurodegeneration markers such as β-amyloid (1–42), tau-p-S199, and tau-p-S404. Melatonin attenuated the manifestation of type II diabetes and reduced oxidative stress, deactivated eIF2α, and decreased tau-p-S404 levels in the brain of animals fed a high-fructose diet.







Similar content being viewed by others
References
Pereira RM, Botezelli JD, da Cruz Rodrigues KC, Mekary RA, Cintra DE, Pauli JR, da Silva ASR, Ropelle ER et al (2017) Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients 9(4). https://doi.org/10.3390/nu9040405
WHO (2003) Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser 916:1–149. http://whqlibdoc.who.int/trs/WHO_TRS_916.pdf. Accessed 23 Feb 2018
Lowette K, Roosen L, Tack J, Vanden Berghe P (2015) Effects of high-fructose diets on central appetite signaling and cognitive function. Front Nutr 2:5. https://doi.org/10.3389/fnut.2015.00005
Aragno M, Mastrocola R (2017) Dietary sugars and endogenous formation of advanced glycation Endproducts: emerging mechanisms of disease. Nutrients 9(4). https://doi.org/10.3390/nu9040385
Jagua Gualdrón A, Ávila Ávila V (2007) Insulin and Alzheimer disease: type 3 diabetes? Rev Fac Med Univ Nac Colomb 55:66–70
Clark IA, Vissel B (2013) Treatment implications of the altered cytokine-insulin axis in neurodegenerative disease. Biochem Pharmacol 86(7):862–871. https://doi.org/10.1016/j.bcp.2013.07.030
De Felice FG, Lourenco MV, Ferreira ST (2014) How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement 10(1 Suppl):S26–S32. https://doi.org/10.1016/j.jalz.2013.12.004
de la Monte SM, Tong M (2014) Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol 88(4):548–559. https://doi.org/10.1016/j.bcp.2013.12.012
Ferreira ST, Clarke JR, Bomfim TR, De Felice FG (2014) Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement 10(1 Suppl):S76–S83. https://doi.org/10.1016/j.jalz.2013.12.010
Agrawal R, Gomez-Pinilla F (2012) ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol 590(10):2485–2499. https://doi.org/10.1113/jphysiol.2012.230078
Lopes A, Vilela TC, Taschetto L, Vuolo F, Petronilho F, Dal-Pizzol F, Streck EL, Ferreira GC et al (2014) Evaluation of the effects of fructose on oxidative stress and inflammatory parameters in rat brain. Mol Neurobiol 50(3):1124–1130. https://doi.org/10.1007/s12035-014-8676-y
Wu A, Ying Z, Gomez-Pinilla F (2004) The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 19(7):1699–1707. https://doi.org/10.1111/j.1460-9568.2004.03246.x
Micó CC, Oliva SB, Tormo GS (2010) Estrés oxidativo en las enfermedades neurodegenerativas. In: Pascual-Leone AM, Medina JM (eds) Monografía XXIX: Acción de las hormonas a nivel cerebral. RANF, Madrid, pp. 283–302
Andersen JK (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Med 10(Suppl):S18–S25. https://doi.org/10.1038/nrn1434
Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res 54(3):245–257. https://doi.org/10.1111/jpi.12010
Lin L, Huang QX, Yang SS, Chu J, Wang JZ, Tian Q (2013) Melatonin in Alzheimer’s disease. Int J Mol Sci 14(7):14575–14593. https://doi.org/10.3390/ijms140714575
Naples M, Baker C, Lino M, Iqbal J, Hussain MM, Adeli K (2012) Ezetimibe ameliorates intestinal chylomicron overproduction and improves glucose tolerance in a diet-induced hamster model of insulin resistance. Am J Physiol Gastrointest Liver Physiol 302(9):G1043–G1052. https://doi.org/10.1152/ajpgi.00250.2011
Wang Y, Kayoumu A, Lu G, Xu P, Qiu X, Chen L, Qi R, Huang S et al (2016) Experimental models in Syrian golden hamster replicate human acute pancreatitis. Sci Rep 6:28014. https://doi.org/10.1038/srep28014
Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K (2000) Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem 275(12):8416–8425
Kasim-Karakas SE, Vriend H, Almario R, Chow LC, Goodman MN (1996) Effects of dietary carbohydrates on glucose and lipid metabolism in golden Syrian hamsters. J Lab Clin Med 128(2):208–213
Bhathena J, Kulamarva A, Martoni C, Urbanska AM, Malhotra M, Paul A, Prakash S (2011) Diet-induced metabolic hamster model of nonalcoholic fatty liver disease. Diabetes Metab Obes 4:195–203. https://doi.org/10.2147/DMSO.S18435
Russell JC, Proctor SD (2006) Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol 15(6):318–330. https://doi.org/10.1016/j.carpath.2006.09.001
Liu GL, Fan LM, Redinger RN (1991) The association of hepatic apoprotein and lipid metabolism in hamsters and rats. Comp Biochem Physiol A Comp Physiol 99(1–2):223–228
Bravo E, Cantafora A, Calcabrini A, Ortu G (1994) Why prefer the golden Syrian hamster (Mesocricetus auratus) to the Wistar rat in experimental studies on plasma lipoprotein metabolism? Comp Biochem Physiol B Comp Biochem 107(2):347–355. https://doi.org/10.1016/0305-0491(94)90058-2
Dillard A, Matthan NR, Lichtenstein AH (2010) Use of hamster as a model to study diet-induced atherosclerosis. Nutr Metab (Lond) 7:89. https://doi.org/10.1186/1743-7075-7-89
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Gerard-Monnier D, Erdelmeier I, Regnard K, Moze-Henry N, Yadan JC, Chaudiere J (1998) Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol 11(10):1176–1183. https://doi.org/10.1021/tx9701790
Martin JP Jr, Dailey M, Sugarman E (1987) Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys 255(2):329–336
Lubinsky S, Bewley GC (1979) Genetics of catalase in DROSOPHILA MELANOGASTER: rates of synthesis and degradation of the enzyme in flies Aneuploid and Euploid for the structural gene. Genetics 91(4):723–742
de Gonzalo-Calvo D, Neitzert K, Fernandez M, Vega-Naredo I, Caballero B, Garcia-Macia M, Suarez FM, Rodriguez-Colunga MJ et al (2010) Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic Biol Med 49(5):733–737. https://doi.org/10.1016/j.freeradbiomed.2010.05.019
Arnao MB, Cano AC, Acosta A (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73(2):239–244. https://doi.org/10.1016/S0308-8146(00)00324-1
Bidwell AJ (2017) Chronic fructose ingestion as a major health concern: is a sedentary lifestyle making it worse? A review. Nutrients 9(6). https://doi.org/10.3390/nu9060549
Cigliano L, Spagnuolo MS, Crescenzo R, Cancelliere R, Iannotta L, Mazzoli A, Liverini G, Iossa S (2017) Short-term fructose feeding induces inflammation and oxidative stress in the hippocampus of young and adult rats. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0518-2
Kitagawa A, Ohta Y, Ohashi K (2012) Melatonin improves metabolic syndrome induced by high fructose intake in rats. J Pineal Res 52(4):403–413. https://doi.org/10.1111/j.1600-079X.2011.00955.x
Dolan LC, Potter SM, Burdock GA (2010) Evidence-based review on the effect of normal dietary consumption of fructose on blood lipids and body weight of overweight and obese individuals. Crit Rev Food Sci Nutr 50(10):889–918. https://doi.org/10.1080/10408398.2010.512990
Park JH, Kho MC, Kim HY, Ahn YM, Lee YJ, Kang DG, Lee HS (2015) Blackcurrant suppresses metabolic syndrome induced by high-fructose diet in rats. Evid Based Complement Alternat Med 2015:1–11. https://doi.org/10.1155/2015/385976
Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D et al (2009) The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab 9(3):252–264. https://doi.org/10.1016/j.cmet.2009.01.011
Kaneko YK, Ishikawa T (2015) Diacylglycerol signaling pathway in pancreatic beta-cells: an essential role of diacylglycerol kinase in the regulation of insulin secretion. Biol Pharm Bull 38(5):669–673. https://doi.org/10.1248/bpb.b15-00060
Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ (1989) Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr 49(5):832–839
Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M et al (2009) Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 30(1):96–116. https://doi.org/10.1210/er.2008-0033
Hardeland R (2017) Melatonin and neuroinflammation: encouraging findings vs. fundamental problems. In: Catala A (ed) Pineal gland: Research advances and clinical challenges. Nova Science, Hauppauge, pp. 163–204
Seneff S, Wainwright G, Mascitelli L (2011) Nutrition and Alzheimer’s disease: the detrimental role of a high carbohydrate diet. Eur J Intern Med 22(2):134–140. https://doi.org/10.1016/j.ejim.2010.12.017
Putakala M, Gujjala S, Nukala S, Desireddy S (2017) Beneficial effects of Phyllanthus amarus against high fructose diet induced insulin resistance and hepatic oxidative stress in male Wistar rats. Appl Biochem Biotechnol 183:744–764. https://doi.org/10.1007/s12010-017-2461-0
Zha BS, Zhou H (2012) ER stress and lipid metabolism in adipocytes. Biochem Res Int 2012:312943–312949. https://doi.org/10.1155/2012/312943
Ariyasu D, Yoshida H, Hasegawa Y (2017) Endoplasmic reticulum (ER) stress and endocrine disorders. Int J Mol Sci 18(2). https://doi.org/10.3390/ijms18020382
Ren LP, Chan SM, Zeng XY, Laybutt DR, Iseli TJ, Sun RQ, Kraegen EW, Cooney GJ et al (2012) Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance. PLoS One 7(2):e30816. https://doi.org/10.1371/journal.pone.0030816
Egawa N, Yamamoto K, Inoue H, Hikawa R, Nishi K, Mori K, Takahashi R (2011) The endoplasmic reticulum stress sensor, ATF6alpha, protects against neurotoxin-induced dopaminergic neuronal death. J Biol Chem 286(10):7947–7957. https://doi.org/10.1074/jbc.M110.156430
Hashida K, Kitao Y, Sudo H, Awa Y, Maeda S, Mori K, Takahashi R, Iinuma M et al (2012) ATF6alpha promotes astroglial activation and neuronal survival in a chronic mouse model of Parkinson’s disease. PLoS One 7(10):e47950. https://doi.org/10.1371/journal.pone.0047950
Ottum MS, Mistry AM (2015) Advanced glycation end-products: modifiable environmental factors profoundly mediate insulin resistance. J Clin Biochem Nutr 57(1):1–12. https://doi.org/10.3164/jcbn.15-3
Rasheed Z, Haqqi TM (2012) Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2alpha, p38-MAPK and NF-kappaB in advanced glycation end products stimulated human chondrocytes. Biochim Biophys Acta 1823(12):2179–2189. https://doi.org/10.1016/j.bbamcr.2012.08.021
Whitcomb EA, Chiu CJ, Taylor A (2015) Dietary glycemia as a determinant of health and longevity. Mol Asp Med 46:14–20. https://doi.org/10.1016/j.mam.2015.08.005
Otoda T, Takamura T, Misu H, Ota T, Murata S, Hayashi H, Takayama H, Kikuchi A et al (2013) Proteasome dysfunction mediates obesity-induced endoplasmic reticulum stress and insulin resistance in the liver. Diabetes 62(3):811–824. https://doi.org/10.2337/db11-1652
Tsakiri EN, Iliaki KK, Hohn A, Grimm S, Papassideri IS, Grune T, Trougakos IP (2013) Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radic Biol Med 65:1155–1163. https://doi.org/10.1016/j.freeradbiomed.2013.08.186
Aijala M, Malo E, Ukkola O, Bloigu R, Lehenkari P, Autio-Harmainen H, Santaniemi M, Kesaniemi YA (2013) Long-term fructose feeding changes the expression of leptin receptors and autophagy genes in the adipose tissue and liver of male rats: a possible link to elevated triglycerides. Genes Nutr 8(6):623–635. https://doi.org/10.1007/s12263-013-0357-3
Baena M, Sanguesa G, Hutter N, Sanchez RM, Roglans N, Laguna JC, Alegret M (2015) Fructose supplementation impairs rat liver autophagy through mTORC activation without inducing endoplasmic reticulum stress. Biochim Biophys Acta 1851(2):107–116. https://doi.org/10.1016/j.bbalip.2014.11.003
Stephan BC, Wells JC, Brayne C, Albanese E, Siervo M (2010) Increased fructose intake as a risk factor for dementia. J Gerontol A Biol Sci Med Sci 65(8):809–814. https://doi.org/10.1093/gerona/glq079
Manzano-Leon N, Mas-Oliva J (2006) Oxidative stress, beta-amyloide peptide and Alzheimer’s disease. Gac Med Mex 142(3):229–238
García T, Jay D (2004) Phosphorylation of tau and Alzheimer’s disease. Gac Med Mex 140:329–333
Evans DB, Rank KB, Bhattacharya K, Thomsen DR, Gurney ME, Sharma SK (2000) Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau's ability to promote microtubule assembly. J Biol Chem 275(32):24977–24983. https://doi.org/10.1074/jbc.M000808200
Rankin CA, Sun Q, Gamblin TC (2008) Pre-assembled tau filaments phosphorylated by GSK-3b form large tangle-like structures. Neurobiol Dis 31(3):368–377. https://doi.org/10.1016/j.nbd.2008.05.011
Cardinali DP, Bernasconi PA, Reynoso R, Toso CF, Scacchi P (2013) Melatonin may curtail the metabolic syndrome: studies on initial and fully established fructose-induced metabolic syndrome in rats. Int J Mol Sci 14(2):2502–2514. https://doi.org/10.3390/ijms14022502
Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, Higgins J, Melanson EL et al (2015) Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr Biol 25(22):3004–3010. https://doi.org/10.1016/j.cub.2015.10.011
McMullan CJ, Curhan GC, Schernhammer ES, Forman JP (2013) Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am J Epidemiol 178(2):231–238. https://doi.org/10.1093/aje/kws470
Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M (2014) Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37(10):1715–1719. https://doi.org/10.5665/sleep.4088
Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R et al (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41(1):82–88. https://doi.org/10.1038/ng.288
Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Ostman B et al (2016) Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab 23(6):1067–1077. https://doi.org/10.1016/j.cmet.2016.04.009
Jin BK, Shin DY, Jeong MY, Gwag MR, Baik HW, Yoon KS, Cho YH, Joo WS et al (1998) Melatonin protects nigral dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP+) neurotoxicity in rats. Neurosci Lett 245(2):61–64
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61(3):253–278. https://doi.org/10.1111/jpi.12360
Acknowledgements
This work was supported by the Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiveness) under grants RD12/0043/0030, RD12/0043/0017, PI13/02741 and PI17/02009; and the Government of the Principality of Asturias under grant GRUPIN14-071, all of them co-financed by the European Regional Development Fund. J.C.B.M. acknowledges his MSc thesis award from AINDACE (Ayuda a la Investigación del Daño Cerebral) foundation (Spain). M.R.M.G acknowledges her postdoctoral fellowship (2013-2586/001-001-EMA2) from the PUEDES Program (European Commission). Y.P. acknowledges her predoctoral fellow (FI14/00405) from the Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiveness).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Oviedo University Local Animal Care and Use Committee approved the experimental protocol. All experiments were carried out according to the Spanish Government Guide and the European Community Guide for Animal Care.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bermejo-Millo, J.C., Guimarães, M.R.M., de Luxán-Delgado, B. et al. High-Fructose Consumption Impairs the Redox System and Protein Quality Control in the Brain of Syrian Hamsters: Therapeutic Effects of Melatonin. Mol Neurobiol 55, 7973–7986 (2018). https://doi.org/10.1007/s12035-018-0967-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0967-2