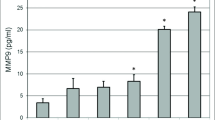
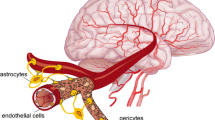
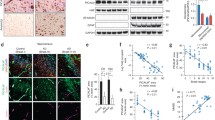
Abstract
Poor Mg status is a risk factor for Alzheimer’s disease (AD), and the underlying mechanisms remain elusive. Here, we provided the first evidence that elevated Mg levels significantly reduced the blood-brain barrier (BBB) permeability and regulated its function in vitro. Transient receptor potential melastatin 7 (TRPM7) and magnesium transporter subtype 1 (MagT1) were two major cellular receptors mediating entry of extracellular Mg2+ into the cells. Elevated Mg levels also induced an accelerated clearance of amyloid-β peptide (Aβ) from the brain to the blood side via BBB transcytosis through low-density lipoprotein receptor-related protein (LRP) and phosphatidylinositol binding clathrin assembly protein (PICALM), while reduced the influx of Aβ from the blood to the brain side involving receptor for advanced glycation end products (RAGE) and caveolae. Mg enhanced BBB barrier properties and overall expression of LRP1 and PICALM whereas reduced that of RAGE and caveolin-1. Apical-to-basolateral and vice versa steady-state Aβ flux achieved an equilibrium of 18 and 0.27 fmol/min/cm2, respectively, about 30 min after the initial addition of physiological levels of free Aβ. Knockdown of caveolin-1 or disruption of caveolae membrane microdomains reduced RAGE-mediated influx significantly, but not LRP1-mediated efflux of Aβ. Stimulating endothelial cells with vascular endothelial growth factor (VEGF) enhanced caveolin-1 phosphorylation and RAGE expression. Co-immunoprecipitation demonstrated that RAGE, but not LRP1, was physically associated with caveolin-1. Thus, Mg can reduce BBB permeability and promote BBB clearance of Aβ from the brain by increasing the expression of LRP1 and PICALM while reducing the level of RAGE and caveolin-1.






Similar content being viewed by others
References
Barbagallo M, Belvedere M, Di Bella G, Dominguez LJ (2011) Altered ionized magnesium levels in mild-to-moderate Alzheimer’s disease. Magnes Res 24(3):S115–S121. https://doi.org/10.1684/mrh.2011.0287
Cilliler AE, Ozturk S, Ozbakir S (2007) Serum magnesium level and clinical deterioration in Alzheimer’s disease. Gerontology 53(6):419–422. https://doi.org/10.1159/000110873
Bostrom F, Hansson O, Gerhardsson L, Lundh T, Minthon L, Stomrud E, Zetterberg H, Londos E (2009) CSF Mg and Ca as diagnostic markers for dementia with Lewy bodies. Neurobiol Aging 30(8):1265–1271. https://doi.org/10.1016/j.neurobiolaging.2007.10.018
Sontia B, Touyz RM (2007) Role of magnesium in hypertension. Arch Biochem Biophys 458(1):33–39. https://doi.org/10.1016/j.abb.2006.05.005
Touyz RM (2004) Magnesium in clinical medicine. Front Biosci 9(1-3):1278–1293. https://doi.org/10.2741/1316
Veronese N, Zanforlini BM, Manzato E, Sergi G (2015) Magnesium and healthy aging. Magnes Res 28(3):112–115. https://doi.org/10.1684/mrh.2015.0387
Ozturk S, Cillier AE (2006) Magnesium supplementation in the treatment of dementia patients. Med Hypotheses 67(5):1223–1225. https://doi.org/10.1016/j.mehy.2006.04.047
Veronese N, Zurlo A, Solmi M, Luchini C, Trevisan C, Bano G, Manzato E, Sergi G et al (2016) Magnesium status in Alzheimer’s disease: a systematic review. Am J Alzheimers Dis Other Demen 31(3):208–213. https://doi.org/10.1177/1533317515602674
Li W, Yu J, Liu Y, Huang X, Abumaria N, Zhu Y, Huang X, Xiong W et al (2014) Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol Brain 7(1):65. https://doi.org/10.1186/s13041-014-0065-y
Xu ZP, Li L, Bao J, Wang ZH, Zeng J, Liu EJ, Li XG, Huang RX et al (2014) Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS One 9(9):e108645. https://doi.org/10.1371/journal.pone.0108645
Wang P, Yu X, Guan PP, Guo JW, Wang Y, Zhang Y, Zhao H, Wang ZY (2015) Magnesium ion influx reduces neuroinflammation in Abeta precursor protein/Presenilin 1 transgenic mice by suppressing the expression of interleukin-1beta. Cell Mol Immunol 14(5):451–464. https://doi.org/10.1038/cmi.2015.93
Jia S, Liu Y, Shi Y, Ma Y, Hu Y, Wang M, Li X (2016) Elevation of brain magnesium potentiates neural stem cell proliferation in the hippocampus of young and aged mice. J Cell Physiol 231(9):1903–1912. https://doi.org/10.1002/jcp.25306
Yu J, Sun M, Chen Z, Lu J, Liu Y, Zhou L, Xu X, Fan D et al (2010) Magnesium modulates amyloid-beta protein precursor trafficking and processing. J Alzheimer’s Dis: JAD 20(4):1091–1106. https://doi.org/10.3233/JAD-2010-091444
Zhou H, Clapham DE (2009) Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci U S A 106(37):15750–15755. https://doi.org/10.1073/pnas.0908332106
Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G et al (2011) Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 475(7357):471–476. https://doi.org/10.1038/nature10246
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163(5):1064–1078. https://doi.org/10.1016/j.cell.2015.10.067
Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B (2000) Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med 6(7):718–718
Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN et al (1998) Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med 4(4):447–451. https://doi.org/10.1038/nm0498-447
Xu H, Sweeney D, Wang R, Thinakaran G, Lo AC, Sisodia SS, Greengard P, Gandy S (1997) Generation of Alzheimer beta-amyloid protein in the trans-Golgi network in the apparent absence of vesicle formation. Proc Natl Acad Sci U S A 94(8):3748–3752. https://doi.org/10.1073/pnas.94.8.3748
Deane R, Yan SD, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L et al (2003) RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9(7):907–913. https://doi.org/10.1038/Nm890
Deane R, Wu ZH, Sagare A, Davis J, Yan SD, Hamm K, Xu F, Parisi M et al (2004) LRP/amyloid beta-peptide interaction mediates differential brain efflux of A beta isoforms. Neuron 43(3):333–344. https://doi.org/10.1016/j.neuron.2004.07.017
Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA et al (2000) Clearance of Alzheimer’s amyloid-beta(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Investig 106(12):1489–1499. https://doi.org/10.1172/JCI10498
Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G (2012) LRP1 in brain vascular smoJ Neuroscioth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. 32(46):16458–16465. https://doi.org/10.1523/JNEUROSCI.3987-12.2012
Zerbinatti CV, Bu G (2005) LRP and Alzheimer’s disease. Rev Neurosci 16(2):123–135
Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song XM, Zlokovic BV (2004) Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med 10(12):1379–1383. https://doi.org/10.1038/Nm1122
Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D et al (1998) Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1-40—asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Investig 102(4):734–743. https://doi.org/10.1172/JCI2029
Wu ZH, Guo H, Chow NW, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S et al (2005) Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med 11(9):959–965. https://doi.org/10.1038/Nm1287
Nishitani WS, Alencar AM, Wang Y (2015) Rapid and localized mechanical stimulation and adhesion assay: TRPM7 involvement in calcium signaling and cell adhesion. PLoS One 10(5):e0126440. https://doi.org/10.1371/journal.pone.0126440
Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, Galla HJ (1998) Hydrocortisone reinforces the blood-brain barrier properties in a serum free cell culture system. Biochem Biophys Res Commun 247(2):312–315
Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A et al (2005) Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 19(13):1872–1874. https://doi.org/10.1096/fj.04-3458fje
Nazer B, Hong S, Selkoe DJ (2008) LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis 30(1):94–102. https://doi.org/10.1016/j.nbd.2007.12.005
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML et al (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid A beta(42) in humans. Ann Neurol 59(3):512–519. https://doi.org/10.1002/Ana.20730
He Y, Yao G, Savoia C, Touyz RM (2005) Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res 96(2):207–215. https://doi.org/10.1161/01.RES.0000152967.88472.3e
Baldoli E, Maier JA (2012) Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis 15(1):47–57. https://doi.org/10.1007/s10456-011-9242-0
Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, van Leeuwen FN, Touyz RM (2010) Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 56(3):453–462. https://doi.org/10.1161/HYPERTENSIONAHA.110.152058
Sontia B, Montezano AC, Paravicini T, Tabet F, Touyz RM (2008) Downregulation of renal TRPM7 and increased inflammation and fibrosis in aldosterone-infused mice: effects of magnesium. Hypertension 51(4):915–921. https://doi.org/10.1161/HYPERTENSIONAHA.107.100339
Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM (2011) Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J 75(2):237–245. https://doi.org/10.1253/circj.CJ-10-1021
Li FY, Lenardo MJ, Chaigne-Delalande B (2011) Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency. Magnes Res 24(3):S109–S114. https://doi.org/10.1684/mrh.2011.0286
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD et al (2017) Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94(3):581–594e585. https://doi.org/10.1016/j.neuron.2017.03.043
Candela P, Gosselet F, Saint-Pol J, Sevin E, Boucau MC, Boulanger E, Cecchelli R, Fenart L (2010) Apical-to-basolateral transport of amyloid-beta peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J Alzheimer’s Dis: JAD 22(3):849–859. https://doi.org/10.3233/JAD-2010-100462
Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A et al (2015) Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat Neurosci 18(7):978–987. https://doi.org/10.1038/nn.4025
Podar K, Shringarpure R, Tai YT, Simoncini M, Sattler M, Ishitsuka K, Richardson PG, Hideshima T et al (2004) Caveolin-1 is required for vascular endothelial growth factor-triggered multiple myeloma cell migration and is targeted by bortezomib. Cancer Res 64(20):7500–7506. https://doi.org/10.1158/0008-5472.CAN-04-0124
Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM (2010) Vascular biology of magnesium and its transporters in hypertension. Magnes Res 23(4):S207–S215. https://doi.org/10.1684/mrh.2010.0222
Sun Y, Sukumaran P, Schaar A, Singh BB (2015) TRPM7 and its role in neurodegenerative diseases. Channels 9(5):253–261. https://doi.org/10.1080/19336950.2015.1075675
Zeng Z, Inoue K, Sun H, Leng T, Feng X, Zhu L, Xiong ZG (2015) TRPM7 regulates vascular endothelial cell adhesion and tube formation. Am J Physiol Cell Physiol 308(4):C308–C318. https://doi.org/10.1152/ajpcell.00275.2013
Baldoli E, Castiglioni S, Maier JA (2013) Regulation and function of TRPM7 in human endothelial cells: TRPM7 as a potential novel regulator of endothelial function. PLoS One 8(3):e59891. https://doi.org/10.1371/journal.pone.0059891
Feske S, Skolnik EY, Prakriya M (2012) Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol 12(7):532–547. https://doi.org/10.1038/nri3233
Zhang Y, Xu J, Ruan YC, Yu MK, O’Laughlin M, Wise H, Chen D, Tian L et al (2016) Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med 22(10):1160–1169. https://doi.org/10.1038/nm.4162
Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G et al (2008) The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem 283(50):34554–34562. https://doi.org/10.1074/jbc.M801487200
Lajoie P, Goetz JG, Dennis JW, Nabi IR (2009) Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol 185(3):381–385. https://doi.org/10.1083/jcb.200811059
Orlowski S, Martin S, Escargueil A (2006) P-glycoprotein and ‘lipid rafts’: some ambiguous mutual relationships (floating on them, building them or meeting them by chance?). Cell Mol Life Sci 63(9):1038–1059. https://doi.org/10.1007/s00018-005-5554-9
Boucher P, Liu PS, Gotthardt M, Hiesberger T, Anderson RGW, Herz J (2002) Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J Biol Chem 277(18):15507–15513. https://doi.org/10.1074/jbc.M200428200
Lisanti MP, Scherer PE, Vidugiriene J, Tang ZL, Hermanowskivosatka A, Tu YH, Cook RF, Sargiacomo M (1994) Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source-implications for human-disease. J Cell Biol 126(1):111–126. https://doi.org/10.1083/jcb.126.1.111
Perrone L, Peluso G, Ab Melone M (2008) RAGE recycles at the plasma membrane in S100B secretory vesicles and promotes Schwann cells morphological logical changes. J Cell Physiol 217(1):60–71. https://doi.org/10.1002/Jcp.21474
Barakat S, Demeule M, Pilorget A, Regina A, Gingras D, Baggetto LG, Beliveau R (2007) Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J Neurochem 101(1):1–8. https://doi.org/10.1111/j.1471-4159.2006.04410.x
Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R (2003) Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell 14(1):334–347. https://doi.org/10.1091/mbc.E02-07-0379
Gonzalez E, Nagiel A, Lin A, Golan DE, Michel T (2004) siRNA-mediated down-regulation of caveolin-1 selectively modulates kinase pathways in endothelial cells. FASEB J 18(8):C258–C258
Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T (2004) Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem 279(39):40659–40669. https://doi.org/10.1074/jbc.M407051200
Acknowledgements
We thank Dr. Yadong Wang from the Cornell University for his advice on experimental design and manuscript preparations. We also thank Drs. Berislav V. Zlokovic, Abhay Sagare, and Zhen Zhao from the University of Southern California as well as Dr. Hongmei Li from the Mayo Clinic for their constructive comments on the study and manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work was supported by the National Institutes of Health [grant number SC3GM113762].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, D., Su, Y., Fu, B. et al. Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-β Transcytosis. Mol Neurobiol 55, 7118–7131 (2018). https://doi.org/10.1007/s12035-018-0896-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0896-0