Abstract
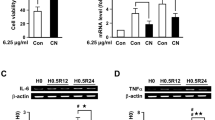
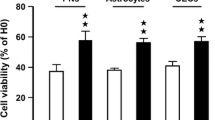
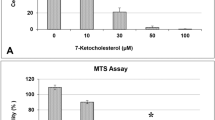
Clinacanthus nutans Lindau (C. nutans) is a traditional herbal medicine widely used in Asian countries for treating a number of remedies including snake and insect bites, skin rashes, viral infections, and cancer. However, the underlying molecular mechanisms for its action and whether C. nutans can offer protection on stroke damage in brain remain largely unknown. In the present study, we demonstrated protective effects of C. nutans extract to ameliorate neuronal apoptotic death in the oxygen-glucose deprivation model and to reduce infarction and mitigate functional deficits in the middle cerebral artery occlusion model, either administered before or after hypoxic/ischemic insult. Using pharmacological antagonist and siRNA knockdown approaches, we demonstrated ability for C. nutans extract to protect neurons and ameliorate ischemic injury through promoting the anti-apoptotic activity of peroxisome proliferator-activated receptor-gamma (PPAR-γ), a stress-induced transcription factor. Reporter and chromatin immunoprecipitation promoter analysis further revealed C. nutans extract to selectively increase CCAAT/enhancer binding protein (C/EBP)β binding to specific C/EBP binding site (-332~-325) on the PPAR-γ promoter to augment its transcription. In summary, we report a novel transcriptional activation involving C/EBPβ upregulation of PPAR-γ expression to suppress ischemic neuronal apoptosis and brain infarct. Recognition of C. nutans to enhance the C/EBPβ → PPAR-γ neuroprotective signaling pathway paves a new way for future drug development for prevention and treatment of ischemic stroke and other neurodegenerative diseases.






Similar content being viewed by others
Abbreviations
- ACO:
-
Acyl-CoA oxidase
- C. nutans :
-
Clinacanthus nutans Lindau
- CN:
-
80% ethanol leaf extract of C. nutans
- CNS:
-
Central nervous system
- C/EBP:
-
CCAAT/enhancer-binding protein
- ChIP:
-
Chromatin immunoprecipitation
- DMSO:
-
Dimethyl sulfoxide
- β-Gal:
-
β-Galactosidase
- icv:
-
Intracerebroventricular
- i.p.:
-
Intraperitoneal
- MCA:
-
Middle cerebral artery
- MRI:
-
Magnetic resonance imaging
- OGD:
-
Oxygen-glucose deprivation
- PNs:
-
Primary cortical neurons
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-gamma
- PPRE:
-
PPAR response element
- tPA:
-
Tissue-type plasminogen activator
References
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR et al, American Heart Association Statistics Committee; Stroke Statistics Subcommittee (2016) Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133(4):e38–360
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67(2):181–198
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24):1581–1587
Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH (2003) Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 9(10):1313–1317
Chen YC, Wu JS, Yang ST, Huang CY, Chang C, Sun GY, Lin TN (2012) Stroke, angiogenesis and phytochemicals. Front Biosci (Schol Ed) 4:599–610
Goetz ME, Judd SE, Hartman TJ, McClellan W, Anderson A, Vaccarino V (2016) Flavanone intake is inversely associated with risk of incident ischemic stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. J Nutr 146(11):2233–2243
Kim J, Fann DY, Seet RC, Jo DG, Mattson MP, Arumugam TV (2016) Phytochemicals in ischemic stroke. NeuroMolecular Med 18(3):283–305
Lee J, Jo DG, Park D, Chung HY, Mattson MP (2014) Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol Rev 66(3):815–868
Sun AY, Wang Q, Simonyi A, Sun GY (2008) Botanical phenolics and brain health. NeuroMolecular Med 10(4):259–274
Alam A, Ferdosh S, Ghafoor K, Hakim A, Juraimi AS, Khatib A, Sarker ZI (2016) Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry. Asian Pac J Trop Med 9(4):402–409
Zulkipli IN, Rajabalaya R, Idris A, Sulaiman NA, David SR (2017) Clinacanthus nutans: a review on ethnomedicinal uses, chemical constituents and pharmacological properties. Pharm Biol 55(1):1093–1113
Ng PY, Chye SM, Ng CH, Koh RY, Tiong YL, Pui LP, Tan YH, Lim CS et al (2017) Clinacanthus nutans hexane extracts induce apoptosis through a caspase-dependent pathway in human cancer cell lines. Asian Pac J Cancer Prev 18(4):917–926
Tan CS, Ho CF, Heng SS, Wu JS, Tan BK, Ng YK, Sun GY, Lin TN et al (2016) Clinacanthus nutans extracts modulate epigenetic link to cytosolic phospholipase A2 expression in SH-SY5Y cells and primary neurons. NeuroMolecular Med 18(3):441–452
Tsai HD, Wu JS, Kao MH, Chen JJ, Sun GY, Ong WY, Lin TN (2016) Clinacanthus nutans protects cortical neurons against hypoxia-induced toxicity by downregulating HDAC1/6. NeuroMolecular Med 18(3):274–282
Lee JE, Ge K (2014) Transcriptional and epigenetic regulation of PPAR-γ expression during adipogenesis. Cell Biosci 4:29
Schweizer S, Meisel A, Märschenz S (2013) Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab 33(9):1335–1346
Goldberg MP, Choi DW (1993) Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci 13(8):3510–3524
Wu JS, Tsai HD, Cheung WM, Hsu CY, Lin TN (2016) PPAR-γ ameliorates neuronal apoptosis and ischemic brain injury via suppressing NF-κB-driven p22phox transcription. Mol Neurobiol 53(6):3626–3645
Huang CY, Chen JJ, Wu JS, Tsai HD, Lin H, Yan YT, Hsu CY, Ho YS et al (2015) Novel link of anti-apoptotic ATF3 with pro-apoptotic CTMP in the ischemic brain. Mol Neurobiol 51(2):543–557
Wu JS, Lin TN, Wu KK (2009) Rosiglitazone and PPAR-γ overexpression protect mitochondrial membrane and prevent mitochondria-mediated apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol 220(1):58–71
Lin TN, Wang CK, Cheung WM, Hsu CY (2000) Induction of angiopoietin and tie receptor mRNA expression following cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 20:387–395
Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY et al (2009) Ligand-activated PPAR-γ protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3ε upregulation. Circulation 119(8):1124–1134
Wu JS, Tsai HD, Huang CY, Chen JJ, Lin TN (2014) 15-deoxy-∆12,14-PGJ2 via activating peroxisome proliferator-activated receptor-gamma suppresses p22phox transcription to protect brain endothelial cells against hypoxia-induced apoptosis. Mol Neurobiol 50(1):221–238
Lin TN, He YY, Wu G, Khan M, Hsu CY (1993) Effect of brain edema on infarct volume in a focal cerebral ischemia model in the rat. Stroke 24:117–121
Lin H, Lin TN, Cheung WM, Nian GM, Tseng PH, Chen SF, Chen JJ, Shyue SK et al (2002) Cyclooxygenase-1 (COX-1) and bicistronic COX-1/prostacyclin synthase gene transfer protect against ischemic cerebral infarction. Circulation 105:1962–1969
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17:472–476
Borlongan CV, Cahill DW, Sanberg PR (1995) Locomotor and passive avoidance deficits following occlusion of the middle cerebral artery. Physiol Behav 58(5):909–917
Metz GA, Whishaw IQ (2002) Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods 115(2):169–179
Lin TN, Sun SW, Cheung WM, Li F, Chang C (2002) Dynamic changes in cerebral blood flow and angiogenesis after transient focal cerebral ischemia in rats: evaluation with serial MRI. Stroke 33:2985–2991
Farsi E, Esmailli K, Shafaei A, Moradi Khaniabadi P, Al Hindi B, Khadeer Ahamed MB, Sandai D, Abdul Sattar M et al (2016) Mutagenicity and preclinical safety assessment of the aqueous extract of Clinacanthus nutans leaves. Drug Chem Toxicol 39(4):461–473
Emsley HC, Hopkins SJ (2008) Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol 7(4):341–353
Ionita CC, Siddiqui AH, Levy EI, Hopkins LN, Snyder KV, Gibbons KJ (2011) Acute ischemic stroke and infections. J Stroke Cerebrovasc Dis 20(1):1–9
Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D (2011) Post-stroke infection: a systematic review and meta-analysis. BMC Neurol 11:110
Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M (2007) Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 69(14):1404–1410
Kongkaew C, Chaiyakunapruk N (2011) Efficacy of Clinacanthus nutans extracts in patients with herpes infection: systematic review and meta-analysis of randomised clinical trials. Complement Ther Med 19(1):47–53
Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T et al (2006) International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58(4):726–741
Chen YC, Wu JS, Tsai HD, Huang CY, Chen JJ, Sun GY, Lin TN (2012) Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol Neurobiol 46(1):114–124
Collino M, Patel NS, Thiemermann C (2008) PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury. Ther Adv Cardiovasc Dis 2(3):179–197
Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN (2010) Anti-apoptotic actions of PPAR-γ against ischemic stroke. Mol Neurobiol 41(2–3):180–186
Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Chen JJ, Liou JY, Shyue SK et al (2006) 15d-Prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 26:481–487
Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE (2009) PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 116(3):205–218
Guo L, Li X, Tang QQ (2015) Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β. J Biol Chem 290(2):755–761
Tsukada J, Yoshida Y, Kominato Y, Auron PE (2011) The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 54(1):6–19
Acknowledgements
We thank the Taiwan Mouse Clinic (TMC; http://tmc.sinica.edu.tw/index.html; MRI: Yu-Ying Tung) for technical support. All authors have read and agreed to the manuscript as written.
Funding Information
This work was supported by grants from the Minister of Science and Technology and Academia Sinica of Taiwan to T.N. Lin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, JS., Kao, MH., Tsai, HD. et al. Clinacanthus nutans Mitigates Neuronal Apoptosis and Ischemic Brain Damage Through Augmenting the C/EBPβ-Driven PPAR-γ Transcription. Mol Neurobiol 55, 5425–5438 (2018). https://doi.org/10.1007/s12035-017-0776-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0776-z