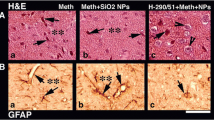
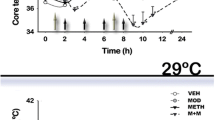
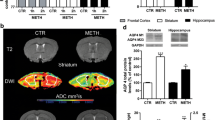
Abstract
The possibility that stress associated with chronic forced swim (FS) may exacerbate methamphetamine (METH) neurotoxicity was examined in a rat model. Rats were subjected to FS in a pool (30 °C) for 15 min daily for 8 days. Control rats were kept at room temperature. METH was administered (9 mg/kg, s.c.) in both control and FS rats and allowed to survive 4 h after the drug injection. METH in FS rats exacerbated BBB breakdown to Evans blue albumin (EBA) by 150 to 220% and [131]-Iodine by 250 to 380% as compared to naive rats after METH. The METH-induced BBB leakage was most pronounced in the cerebral cortex followed by the hippocampus, cerebellum, thalamus, and hypothalamus in both FS and naive rats. The regional BBB changes were associated with a reduction in the local cerebral blood flow (CBF). Brain edema was also higher by 2 to 4% in FS rats after METH than in naive animals. Neuronal and glial cell injuries were aggravated by threefold to fivefold after METH in FS than the control group. Pretreatment with ondansetron (1 mg/kg, i.p.) 30 min before METH injection in naive rats reduced the brain pathology and improved the CBF. However, TiO2-nanowired delivery of ondansetron (1 mg/kg, i.p.) was needed to reduce METH-induced brain damage, BBB leakage, reduction in CBF, and edema formation in FS. Taken together, these observations are the first to show that METH exacerbates BBB breakdown leading to neurotoxicity in FS animals. This effect of METH-induced BBB breakdown and brain pathology in naive and FS rats is attenuated by ondansetron treatment indicating an involvement of 5-HT3 receptors, not reported earlier.



Similar content being viewed by others
References
Koenig JI, Walker CD, Romeo RD, Lupien SJ (2011) Effects of stress across the lifespan. Stress 14(5):475–480. doi:10.3109/10253890.2011.604879
Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A (2011) Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress 14(2):227–232. doi:10.3109/10253890.2010.522279
Yam KY, Naninck EF, Schmidt MV, Lucassen PJ, Korosi A (2015) Early-life adversity programs emotional functions and the neuroendocrine stress system: the contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress 18(3):328–342
Maier LJ, Liechti ME, Herzig F, Schaub MP (2013) To dope or not to dope: neuroenhancement with prescription drugs and drugs of abuse among Swiss university students. PLoS One 8(11):e77967. doi:10.1371/journal.pone.0077967
Cabib S, Puglisi-Allegra S (1996) Stress, depression and the mesolimbic dopamine system. Psychopharmacology 128(4):331–342
Chomchai C, Chomchai S (2015) Global patterns of methamphetamine use. Curr Opin Psychiatry 28(4):269–274. doi:10.1097/YCO.0000000000000168
Courtney KE, Ray LA (2014) Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 143:11–21. doi:10.1016/j.drugalcdep.2014.08.003
Halpin LE, Collins SA, Yamamoto BK (2014) Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci 97(1):37–44. doi:10.1016/j.lfs.2013.07.014
Yu S, Zhu L, Shen Q, Bai X, Di X (2015) Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav Neurol 2015:103969. doi:10.1155/2015/103969
Robinson JD, Howard CD, Pastuzyn ED, Byers DL, Keefe KA, Garris PA (2014) Methamphetamine-induced neurotoxicity disrupts pharmacologically evoked dopamine transients in the dorsomedial and dorsolateral striatum. Neurotox Res 26(2):152–167. doi:10.1007/s12640-014-9459-y
Raineri M, González B, Rivero-Echeto C, Muñiz JA, Gutiérrez ML, Ghanem CI, Cadet JL, García-Rill E et al (2015) Differential effects of environment-induced changes in body temperature on modafinil's actions against methamphetamine-induced striatal toxicity in mice. Neurotox Res 27(1):71–83. doi:10.1007/s12640-014-9493-9
Sharma HS, Kiyatkin EA, Patnaik R, Lafuente JV, Muresanu DF, Sjöquist PO, Sharma A (2015) Exacerbation of methamphetamine neurotoxicity in cold and hot environments: neuroprotective effects of an antioxidant compound H-290/51. Mol Neurobiol 52(2):1023–1033. doi:10.1007/s12035-015-9252-9
O'Shea E, Urrutia A, Green AR, Colado MI (2014) Current preclinical studies on neuroinflammation and changes in blood-brain barrier integrity by MDMA and methamphetamine. Neuropharmacology 87:125–134. doi:10.1016/j.neuropharm.2014.02.015
Sajja RK, Rahman S, Cucullo L (2016) Drugs of abuse and blood-brain barrier endothelial dysfunction: a focus on the role of oxidative stress. J Cereb Blood Flow Metab 36(3):539–554. doi:10.1177/0271678X15616978
Kiyatkin EA, Sharma HS (2015) Not just the brain: methamphetamine disrupts blood-spinal cord barrier and induces acute glial activation and structural damage of spinal cord cells. CNS Neurol Disord Drug Targets 14(2):282–294
Kiyatkin EA, Brown PL, Sharma HS (2007) Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. Eur J Neurosci 26(5):1242–1253
Sharma HS, Ali SF (2006) Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci 1074:198–224
Sharma HS, Kiyatkin EA (2009) Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: an experimental study using light and electron microscopy. J Chem Neuroanat 37(1):18–32. doi:10.1016/j.jchemneu.2008.08.002
Sharma HS, Westman J, Navarro JC, Dey PK, Nyberg F (1995) Probable involvement of serotonin in the increased permeability of the blood-brain barrier by forced swimming. An experimental study using Evans blue and 131I-sodium tracers in the rat. Behav Brain Res 72(1–2):189–196
Sharma HS, Dey PK (1986) Probable involvement of 5-hydroxytryptamine in increased permeability of blood-brain barrier under heat stress in young rats. Neuropharmacology 25(2):161–167
Sharma HS, Dey PK (1986) Influence of long-term immobilization stress on regional blood-brain barrier permeability, cerebral blood flow and 5-HT level in conscious normotensive young rats. J Neurol Sci 72(1):61–76
Sharma HS, Cervós-Navarro J, Dey PK (1991) Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci Res 10(3):211–221
Sharma HS, Dey PK (1987) Influence of long-term acute heat exposure on regional blood-brain barrier permeability, cerebral blood flow and 5-HT level in conscious normotensive young rats. Brain Res 424(1):153–162
Yankelevitch-Yahav R, Franko M, Huly A, Doron R (2015) The forced swim test as a model of depressive-like behavior. J Vis Exp 97. doi:10.3791/52587
Reed AL, Happe HK, Petty F, Bylund DB (2008) Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacology 197(3):433–441. doi:10.1007/s00213-007-1052-0
Stone EA, Lin Y (2011) Open-space forced swim model of depression for mice. Curr Protoc Neurosci Chapter 9:Unit9.36. doi:10.1002/0471142301.ns0936s54
Kuhn M, Popovic A, Pezawas L (2014) Neuroplasticity and memory formation in major depressive disorder: an imaging genetics perspective on serotonin and BDNF. Restor Neurol Neurosci 32(1):25–49. doi:10.3233/RNN-139005
Helton SG, Lohoff FW (2015) Serotonin pathway polymorphisms and the treatment of major depressive disorder and anxiety disorders. Pharmacogenomics 16(5):541–553. doi:10.2217/pgs.15.15
Morrissette DA, Stahl SM (2014) Modulating the serotonin system in the treatment of major depressive disorder. CNS Spectr 19 Suppl 1:57–67; quiz 54-7, 68. doi:10.1017/S1092852914000613
Müller CP, Homberg JR (2015) The role of serotonin in drug use and addiction. Behav Brain Res 277:146–192. doi:10.1016/j.bbr.2014.04.007
Chiu VM, Schenk JO (2012) Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Curr Drug Abuse Rev 5(3):227–242
Doyle JR, Yamamoto BK (2010) Serotonin 2 receptor modulation of hyperthermia, corticosterone, and hippocampal serotonin depletions following serial exposure to chronic stress and methamphetamine. Psychoneuroendocrinology 35(4):629–633. doi:10.1016/j.psyneuen.2009.10.001
Kondo M, Nakamura Y, Ishida Y, Shimada S (2015) The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol Psychiatry 20(11):1428–1437. doi:10.1038/mp.2014.153
Stäubli U, Xu FB (1995) Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci 15(3 Pt 2):2445–2452
Gupta D, Radhakrishnan M, Kurhe Y (2015) Effect of a novel 5-HT3 receptor antagonist 4i, in corticosterone-induced depression-like behavior and oxidative stress in mice. Steroids 96:95–102. doi:10.1016/j.steroids.2015.01.021
Hodge CW, Kelley SP, Bratt AM, Iller K, Schroeder JP, Besheer J (2004) 5-HT(3A) receptor subunit is required for 5-HT3 antagonist-induced reductions in alcohol drinking. Neuropsychopharmacology 29(10):1807–1813
Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, Murphy JM (2010) Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol 44(3):245–255. doi:10.1016/j.alcohol.2010.01.002
Hensler JG, Hodge CW, Overstreet DH (2004) Reduced 5-HT3 receptor binding and lower baseline plus maze anxiety in the alcohol-preferring inbred fawn-hooded rat. Pharmacol Biochem Behav 77(2):281–289
Takamatsu Y, Yamamoto H, Hagino Y, Markou A, Ikeda K (2011) The selective serotonin reuptake inhibitor paroxetine, but not fluvoxamine, decreases methamphetamine conditioned place preference in mice. Curr Neuropharmacol 9(1):68–72. doi:10.2174/157015911795017236
Dimatelis JJ, Russell VA, Stein DJ, Daniels WM (2012) The effects of lobeline and naltrexone on methamphetamine-induced place preference and striatal dopamine and serotonin levels in adolescent rats with a history of maternal separation. Metab Brain Dis 27(3):351–361. doi:10.1007/s11011-012-9288-8
Ginawi OT, Al-Majed AA, Al-Suwailem AK (2005) Ondansetron, a selective 5-HT3 antagonist, antagonizes methamphetamine-induced anorexia in mice. Pharmacol Res 51(3):255–259
Yoo JH, Cho JH, Yu HS, Lee KW, Lee BH, Jeong SM, Nah SY, Kim HC et al (2006) Involvement of 5-HT receptors in the development and expression of methamphetamine-induced behavioral sensitization: 5-HT receptor channel and binding study. J Neurochem 99(3):976–988
Johnson BA, Ait-Daoud N, Elkashef AM, Smith EV, Kahn R, Vocci F, Li SH, Bloch DA (2008) Methamphetamine Study Group. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of methamphetamine dependence. Int J Neuropsychopharmacol 11(1):1–14
Guide for the Care and Use of Laboratory Animals (2011) 8th edition. National Institute of Health, The National Academies Press, Washington DC, www.nap.edu
Sharma HS, Feng L, Lafuente JV, Muresanu DF, Tian ZR, Patnaik R, Sharma A (2015) TiO2-nanowired delivery of mesenchymal stem cells thwarts diabetes- induced exacerbation of brain pathology in heat stroke: an experimental study in the rat using morphological and biochemical approaches. CNS Neurol Disord Drug Targets 14(3):386–399
Tian ZR, Sharma A, Nozari A, Subramaniam R, Lundstedt T, Sharma HS (2012) Nanowired drug delivery to enhance neuroprotection in spinal cord injury. CNS Neurol Disord Drug Targets 11(1):86–95
Sharma A, Menon P, Muresanu DF, Ozkizilcik A, Tian ZR, Lafuente JV, Sharma HS (2016) Nanowired drug delivery across the blood-brain barrier in central nervous system injury and repair. CNS Neurol Disord Drug Targets 15(9):1092–1117
Sharma HS, Olsson Y, Dey PK (1990) Changes in blood-brain barrier and cerebral blood flow following elevation of circulating serotonin level in anesthetized rats. Brain Res 517(1–2):215–223
Johansson B, Li CL, Olsson Y, Klatzo I (1970) The effect of acute arterial hypertension on the blood-brain barrier to protein tracers. Acta Neuropathol 16(2):117–124
Johansson B, Nilsson B (1977) The pathophysiology of the blood-brain barrier dysfunction induced by severe hypercapnia and by epileptic brain activity. Acta Neuropathol 38(2):153–158
Rapoport SI, Fredericks WR, Ohno K, Pettigrew KD (1980) Quantitative aspects of reversible osmotic opening of the blood-brain barrier. Am J Phys 238(5):R421–R431
Fredericks WR, Rapoport SI (1988) Reversible osmotic opening of the blood-brain barrier in mice. Stroke 19(2):266–268
Sokrab TE, Johansson BB, Kalimo H, Olsson Y (1988) A transient hypertensive opening of the blood-brain barrier can lead to brain damage. Extravasation of serum proteins and cellular changes in rats subjected to aortic compression. Acta Neuropathol 75(6):557–565
Rapoport SI (1976) Blood-brain barrier in physiology and medicine. Raven Press, New York
Bradbury MWB (1979) The concept of a blood-brain barrier. John Wiley, Chichester
Sharma HS (1999) Pathophysiology of blood-brain barrier, brain edema and cell injury following hyperthermia: new role of heat shock protein, nitric oxide and carbon monoxide. An experimental study in the rat using light and electron microscopy. Acta Universitatis Upsaliensis 830:1–94
Sharma HS (1987) Effect of captopril (a converting enzyme inhibitor) on blood-brain barrier permeability and cerebral blood flow in normotensive rats. Neuropharmacology 26(1):85–92
Olsson Y, Sharma HS, Pettersson CA (1990) Effects of p-chlorophenylalanine on microvascular permeability changes in spinal cord trauma. An experimental study in the rat using 131I-sodium and lanthanum tracers. Acta Neuropathol 79(6):595–603
Sharma HS, Cervós-Navarro J (1990) Brain oedema and cellular changes induced by acute heat stress in young rats. Acta Neurochir Suppl (Wien) 51:383–386
Marcus ML, Busija DW, Bischof CJ, Heistad DD (1981) Methods for measurement of cerebral blood flow. Fed Proc 40(8):2306–2310
Sadoshima S, Heistad DD (1983) Regional cerebral blood flow during hypotension in normotensive and stroke-prone spontaneously hypertensive rats: effect of sympathetic denervation. Stroke 14(4):575–579
Sharma HS, Zimmer C, Westman J, Cervós-Navarro J (1992) Acute systemic heat stress increases glial fibrillary acidic protein immunoreactivity in brain: experimental observations in conscious normotensive young rats. Neuroscience 48(4):889–901
Sharma HS, Olsson Y, Cervós-Navarro J (1993) p-Chlorophenylalanine, a serotonin synthesis inhibitor, reduces the response of glial fibrillary acidic protein induced by trauma to the spinal cord. An immunohistochemical investigation in the rat. Acta Neuropathol 86(5):422–427
Elliott KA, Jasper H (1949) Measurement of experimentally induced brain swelling and shrinkage. Am J Phys 157(1):122–129
Kiyatkin EA, Sharma HS (2009) Acute methamphetamine intoxication: brain hyperthermia, blood-brain barrier, brain edema, and morphological cell abnormalities. Int Rev Neurobiol 88:65–100. doi:10.1016/S0074-7742(09)88004-5
Kiyatkin EA, Sharma HS (2009) Permeability of the blood-brain barrier depends on brain temperature. Neuroscience 161(3):926–939. doi:10.1016/j.neuroscience.2009.04.004
Sharma HS, Patnaik R, Patnaik S, Mohanty S, Sharma A, Vannemreddy P (2007) Antibodies to serotonin attenuate closed head injury induced blood brain barrier disruption and brain pathology. Ann N Y Acad Sci 1122:295–312
Miller DJ, Balaram P, Young NA, Kaas JH (2014) Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front Neuroanat 8:36. doi:10.3389/fnana.2014.00036
Schmitz C, Hof PR (2000) Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat 20(1):93–114
Collins CE, Young NA, Flaherty DK, Airey DC, Kaas JH (2010) A rapid and reliable method of counting neurons and other cells in brain tissue: a comparison of flow cytometry and manual counting methods. Front Neuroanat 4:5. doi:10.3389/neuro.05.005.2010
Sharma HS, Olsson Y, Cervós-Navarro J (1993) Early perifocal cell changes and edema in traumatic injury of the spinal cord are reduced by indomethacin, an inhibitor of prostaglandin synthesis. Experimental study in the rat. Acta Neuropathol 85(2):145–153
Zimmer C, Sampaolo S, Sharma HS, Cervós-Navarro J (1991) Altered glial fibrillary acidic protein immunoreactivity in rat brain following chronic hypoxia. Neuroscience 40(2):353–361
Sharma HS, Olsson Y, Persson S, Nyberg F (1995) Trauma-induced opening of the blood-spinal cord barrier is reduced by indomethacin, an inhibitor of prostaglandin biosynthesis. Experimental observations in the rat using [131I]-sodium, Evans blue and lanthanum as tracers. Restor Neurol Neurosci 7(4):207–215. doi:10.3233/RNN-1994-7403
Sharma H S, Westman J, Nyberg F (1998) Pathophysiology of brain edema and cell changes following hyperthermic brain injury, In: Sharma H S, Westman J (Eds). Brain Functions in Hot Environment. Progress in Brain Research, 115: 351–412
Sharma HS (2004) Blood-brain and spinal cord barriers in stress. In: Sharma HS, Westman J (eds) The Blood-Spinal Cord and Brain Barriers in Health and Disease. Elsevier Academic Press, San Diego, pp. 231–298
Sharma HS, Westman J (2004) The Blood-Spinal Cord and Brain Barriers in Health and Disease. Academic Press, San Diego, pp. 1–617 (release date: Nov. 9, 2003)
Degenhardt L, Larney S, Chan G, Dobbins T, Weier M, Roxburgh A, Hall WD, McKetin R (2016) Estimating the number of regular and dependent methamphetamine users in Australia, 2002-2014. Med J Aust 204(4):153
Zhuang SM, Chen F (2016) Chinese adolescents and youth with methamphetamine dependence: prevalence and concurrent psychological problems. Nurs Res 65(2):117–124. doi:10.1097/NNR.0000000000000141
Been F, Bijlsma L, Benaglia L, Berset JD, Botero-Coy AM, Castiglioni S, Kraus L, Zobel F et al (2016) Assessing geographical differences in illicit drug consumption—a comparison of results from epidemiological and wastewater data in Germany and Switzerland. Drug Alcohol Depend 161:189–199. doi:10.1016/j.drugalcdep.2016.02.002
Lai FY, O'Brien JW, Thai PK, Hall W, Chan G, Bruno R, Ort C, Prichard J et al (2016) Cocaine, MDMA and methamphetamine residues in wastewater: Consumption trends (2009-2015) in South East Queensland, Australia. Sci Total Environ 568:803–809. doi:10.1016/j.scitotenv.2016.05.181
Hockenhull J, Murphy KG, Paterson S (2016) Mephedrone use is increasing in London. Lancet 387(10029):1719–1720. doi:10.1016/S0140-6736(16)30258-6
World Drug Report (2016) Illicit drug markets. Situation and trends. http://www.unodc.org/doc/wdr2016/WDR_2016_Chapter_1_Drug_use.pdf visited on Oct 22, 2016
McKetin R, Lubman DI, Najman JM, Dawe S, Butterworth P, Baker AL (2014) Does methamphetamine use increase violent behaviour? Evidence from a prospective longitudinal study. Addiction 109(5):798–806. doi:10.1111/add.12474
Brecht ML, Herbeck D (2013) Methamphetamine use and violent behavior: user perceptions and predictors. J Drug Issues 43(4):468–482
Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M (2004) Methamphetamine treatment project. Psychiatric symptoms in methamphetamine users. Am J Addict 13(2):181–190
Cruickshank CC, Dyer KR (2009) A review of the clinical pharmacology of methamphetamine. Addiction 104(7):1085–1099. doi:10.1111/j.1360-0443.2009.02564.x
Ramkissoon A, Wells PG (2015) Methamphetamine oxidative stress, neurotoxicity, and functional deficits are modulated by nuclear factor-E2-related factor 2. Free Radic Biol Med 89:358–368. doi:10.1016/j.freeradbiomed.2015.07.157
Janardhanan R, Kannan A (2016) Methamphetamine cardiotoxicity: unique presentation with multiple bi-ventricular thrombi. Am J Med 129(1):e3–e4. doi:10.1016/j.amjmed.2015.08.006
Yu Q, Larson DF, Watson RR (2003) Heart disease, methamphetamine and AIDS. Life Sci 73(2):129–140
Chin KM, Channick RN, Rubin LJ (2006) Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest 130(6):1657–1663
Jones ES, Rayner BL (2015) Hypertension, end-stage renal disease and mesangiocapillary glomerulonephritis in methamphetamine users. S Afr Med J 105(3):199–201
Herbeck DM, Brecht ML, Lovinger K (2015) Mortality, causes of death, and health status among methamphetamine users. J Addict Dis 34(1):88–100. doi:10.1080/10550887.2014.975610
Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R (2008) Methamphetamine treatment project. Identifying methamphetamine users at risk for major depressive disorder: findings from the methamphetamine treatment project at three-year follow-up. Am J Addict 17(2):99–102. doi:10.1080/10550490701861110
Emmrich JV, Ejaz S, Neher JJ, Williamson DJ, Baron JC (2015) Regional distribution of selective neuronal loss and microglial activation across the MCA territory after transient focal ischemia: quantitative versus semiquantitative systematic immunohistochemical assessment. J Cereb Blood Flow Metab 35(1):20–27. doi:10.1038/jcbfm.2014.181
Gao W, Li J, Cirillo J, Borgens R, Cho Y (2014) Action at a distance: functional drug delivery using electromagnetic-field-responsive polypyrrole nanowires. Langmuir 30(26):7778–7788. doi:10.1021/la500033b
Fischer KE, Alemán BJ, Tao SL, Hugh Daniels R, Li EM, Bünger MD, Nagaraj G, Singh P et al (2009) Biomimetic nanowire coatings for next generation adhesive drug delivery systems. Nano Lett 9(2):716–720. doi:10.1021/nl803219f
Uskoković V, Lee PP, Walsh LA, Fischer KE, Desai TA (2012) PEGylated silicon nanowire coated silica microparticles for drug delivery across intestinal epithelium. Biomaterials 33(5):1663–1672. doi:10.1016/j.biomaterials.2011.11.010
Chu LF, Liang DY, Li X, Sahbaie P, D'arcy N, Liao G, Peltz G, David Clark J (2009) From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics 19(3):193–205. doi:10.1097/FPC.0b013e328322e73d
Reulen HJ, Graham R, Spatz M, Klatzo I (1977) Role of pressure gradients and bulk flow in dynamics of vasogenic brain edema. J Neurosurg 46(1):24–35
Brightman MW, Klatzo I, Olsson Y, Reese TS (1970) The blood-brain barrier to proteins under normal and pathological conditions. J Neurol Sci 10(3):215–239
Klatzo I (1969) Aspects of the blood-brain barrier in brain edema. Clin Neurosurg 16:472–473
Klatzo I (1987) Pathophysiological aspects of brain edema. Acta Neuropathol 72(3):236–239
Kalimo H, Fredriksson K, Nordborg C, Auer RN, Olsson Y, Johansson B (1986) The spread of brain oedema in hypertensive brain injury. Med Biol 64(2–3):133–137
Elliott MB, Jallo JJ, Tuma RF (2008) An investigation of cerebral edema and injury volume assessments for controlled cortical impact injury. J Neurosci Methods 168(2):320–324
Acknowledgements
This study is supported by grants from the Air Force Office of Scientific Research (EOARD, London, UK) and Air Force Material Command, USAF, under grant number FA8655-05-1-3065; the National Institutes of Health (R01 AG028679) Swedish Medical Research Council (Nr 2710-HSS), Göran Gustafsson Foundation, Stockholm, Sweden (HSS), Astra Zeneca, Mölndal, Sweden (HSS/AS), the University Grants Commission, New Delhi, India (HSS/AS), Ministry of Science & Technology, Govt. of India (HSS/AS), Indian Medical Research Council, New Delhi, India (HSS/AS), and India-EU Co-operation Program (RP/AS/HSS) and IT 901/16 (JVL), Government of Basque Country, and UFI 11/32 and PPG 17/51 (JVL) University of Basque Country, Spain, and Society for Neuroprotection and Neuroplasticity (SSNN), Romania. We thank Suraj Sharma, Uppsala, Sweden, for computer and graphic support. The US Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force Office of Scientific Research or the US Government. We are grateful to anonymous reviewers for their very constructive comments that resulted in a significant increase in the quality of the revised manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were carried out according to National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Local Institutional Ethics Committee [44].
Rights and permissions
About this article
Cite this article
Lafuente, J.V., Sharma, A., Muresanu, D.F. et al. Repeated Forced Swim Exacerbates Methamphetamine-Induced Neurotoxicity: Neuroprotective Effects of Nanowired Delivery of 5-HT3-Receptor Antagonist Ondansetron. Mol Neurobiol 55, 322–334 (2018). https://doi.org/10.1007/s12035-017-0744-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0744-7