Abstract
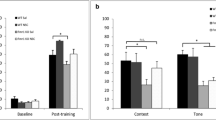
Behavioral intervention therapy has proven beneficial in the treatment of autism and intellectual disabilities (ID), raising the possibility of certain changes in molecular mechanisms activated by these interventions that may promote learning. Fragile X syndrome (FXS) is a neurodevelopmental disorder characterized by autistic features and intellectual disability and can serve as a model to examine mechanisms that promote learning. FXS results from mutations in the fragile X mental retardation 1 gene (Fmr1) that prevents expression of the Fmr1 protein (FMRP), a messenger RNA (mRNA) translation regulator at synapses. Among many other functions, FMRP organizes a complex with the actin cytoskeleton-regulating small Rho GTPase Rac1. As in humans, Fmr1 KO mice lacking FMRP display autistic-like behaviors and deformities of actin-rich synaptic structures in addition to impaired hippocampal learning and synaptic plasticity. These features have been previously linked to proper function of actin remodeling proteins that includes Rac1. An important step in Rac1 activation and function is its translocation to the membrane, where it can influence synaptic actin cytoskeleton remodeling during hippocampus-dependent learning. Herein, we report that Fmr1 KO mouse hippocampus exhibits increased levels of membrane-bound Rac1, which may prevent proper learning-induced synaptic changes. We also determine that increasing training intensity during fear conditioning (FC) training restores contextual memory in Fmr1 KO mice and reduces membrane-bound Rac1 in Fmr1 KO hippocampus. Increased training intensity also results in normalized long-term potentiation in hippocampal slices taken from Fmr1 KO mice. These results point to interventional treatments providing new therapeutic options for FXS-related cognitive dysfunction.













Similar content being viewed by others
References
Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y et al (2001) Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107:477–487
Todd PK, Mack KJ, Malter JS (2003) The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor dependent translation of PSD-95. Proc Natl Acad Sci USA 100:14374–14378
Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C (2003) The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112(3):317–327
Niere F, Wilkerson JR, Huber KM (2012) Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered arc translation and long-term depression. J Neurosci 32(17):5924–5936
Deacon RM, Glass L, Snape M, Hurley MJ, Altimiras FJ, Biekofsky RR, Cogram P (2015) NNZ-2566, a novel analog of (1-3) IGF-1, as a potential therapeutic agent for fragile X syndrome. NeuroMolecular Med 17:71–82
Oddi D, Subashi E, Middei S, Bellocchio L, Lemaire-Mayo V, Guzman M, Crsio WE, D’Mato FR et al (2015) Early social enrichment rescues adult behavioral and brain abnormalities in a mouse model of fragile X syndrome. Neuropsychopharmachology 40(5):1113–1122
Tian M, Zeng Y, Hu Y, Yuan X, Liu S, Li J, Lu P, Sun Y et al (2015) 7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology 89:43–53
Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM (2007) Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci 27(40):10685–10694
Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN et al (2011) Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile x mental retardation protein. Neuron 72(4):630–642
Boda B, Mendez P, Boury-Jamot B, Magara F, Muller D (2014) Reversal of activity-mediated spine dynamics and learning impairment in a mouse model of fragile X syndrome. Eur J Neurosci 39:1130–1137
Ben IE, Zachor DA (2007) The effects of intellectual functioning and autism severity on outcome of early behavioral intervention for children with autism. Res Dev Disabil 28(3):287–303
Howlin P, Magiati I, Charman T (2009) Systematic review of early intensive behavioral interventions for children with autism. Am J Intellect Dev Disabil 114(1):23–41
Seese RR, Wang K, Yao YQ, Lynch G, Gall CM (2014) Spaced training rescues memory and ERK1/2 signaling in fragile X syndrome model mice. Proc Natl Acad Sci U S A 111:16907–16912
Maren S (1998) Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci 18:3088–3097
Megías M, Emri Z, Freund TF, Gulyás AI (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102(3):527–540
Roberts TF, Tschida KA, Klein ME, Mooney R (2010) Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 463:948–952
Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924
Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB (2003) Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130:5543–5552
Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A (2003) CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron 38:887–898
Castets M, Schaeffer C, Bechara E, Schenck A, Khandjian EW, Luche S, Moine H, Rabilloud T et al (2005) FMRP interferes with the Rac1 pathway and controls actin cytoskeleton dynamics in murine fibroblasts. Hum Mol Genet 14(6):835–844
De Rubeis S, Pasciuto E, Li KW, Fernandez E, Di Marino D, Buzzi A, Ostroff LE, Klann E et al (2013) CYFIP1 coordinates mRNA translation and cytoskeleton remodeling to ensure proper dendritic spine formation. Neuron 79:1169–1182
Hotulainen P, Hoogenraad CC (2010) Actin in dendritic spines: connecting dynamics to function. J Cell Biol1 89:619–629
Lamprecht R (2011) The role of the actin cytoskeleton in fear memory formation. Front Behav Neurosci 5:39
Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6(2):167–180
Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L (1997) Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes Mol. Cell Biol 17:1129–1143
Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J (1997) Human p21-activated kinase (PAK1) regulates actin organization in mammalian cells. Curr Biol 7:202–210
Chong C, Tan L, Lim L, Manser E (2001) The mechanism of PAK activation: autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem 276:17347–17353
Moon SY, Zheng Y (2003) Rho GTPase-activating proteins in cell regulation. Trends Cell Biol 13(1):13–22
Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O et al (2008) Rho family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem 283(37):25150–25163
Garcia-Mata R, Boulter E, Burridge K (2011) The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 12(8):493–504
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th edition. Washington (DC): National Academies Press (US); 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK54050/
Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV (2010) Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav 9(6):562–574
Ding Q, Sethna F, Wang H (2014) Behavioral analysis of male and female of Fmr1 KO mice on C57BL/6 background. Behav Brain Res 271:72–78
Nguy S, Tejada-Simon MV (2015) Phenotype analysis and rescue on female FVB.129-Fmr1 knockout mice. Front Biol 11(1):43–52
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bustelo XR, Ojeda V, Barreira M, Sauzeau V, Castro-Castro A (2012) Rac-ing to the plasma membrane: the long and complex work commute of Rac1 during cell signaling. Small GTPases 3:60–66
Yuan M, Gao S, Sun C, Chen L, Shi Q, Hu J, Yu R, Zhou X (2015) Inhibiting geranylgeranyltransferase I activity decrease spine density in central nervous system. Hippocampus 25(3):373–384
Martinez LA, Klann E, Tejada-Simon MV (2007) Translocation and activation of Rac in the hippocampus during associative contextual fear learning. Neuorbiol Learn Mem 88:104–113
de Diego-Otero Y, Romero-Zerbo Y, el Bekay R, Decara J, Sanchez L, Rodriguez-de Fonseca F, del Arco-Herrera I (2009) α-tocopherol protects against oxidative stress in the fragile X knockout mouse: an experimental therapeutic approach for the Fmr1 deficiency. Neuropsychopharmacology 34(4):1011–1026
Bongmba OYN, Martinez LM, Elhardt ME, Butler K, Tejada-Simon MV (2011) Modulation of dendritic spines and synaptic function by Rac1: a possible link to fragile X syndrome pathology. Brain Res 1399:79–95
Majumder P, Chu JF, Chatterjee B, Swamy KB, Shen CJ (2016) Co-regulation of mRNA translation by TDP-43 and fragile X syndrome protein FMRP. Acta Neuropathol 132(5):721–738
Penzes P, Cahill ME, Jones KA, Van Leeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14(3):285–293
Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20:5329–5338
Schuster S, Rivalan M, Strauss U, Stoenica L, Trimbuch T, Rademacher N, Parthasarathy S, Lajkó D et al (2015) NOMA-GAP/ARHGAP33 regulates synapse development and autistic-like behavior in the mouse. Mol Psychiatry 20(9):1120–1131
Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K (2010) Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol 12(5):477–483
Olmos-Serrano JL, Corbin JG, Burns MP (2011) The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev Neurosci 33:395–403
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232(2):331–356
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R (2014) Engineering a memory with LTD and LTP. Nature 511(7509):348–352
Song C, Detert JA, Sehgal M, Moyer JR Jr (2012) Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J Neuorphysiol 107:3397–3408
Zhang H, Web DJ, Asmussen H, Niu S, Horwitz AF (2005) A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci 25:3379–3388
Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ et al (2007) Kalarin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56(4):640–656
Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL (2003) Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the RhoGEF Kalirin. Neuron 37(2):263–274
Winson J (1978) Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201(4351):160–163
Seidenbecher T, Laxmi TR, Stork O, Pape HC (2003) Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 301:846–850
Rotschafer SE, Marshak S, Cramer KS (2015) Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem. PLoS One 10(2)
Xu L, Anwyl R, Rowan MJ (1998) Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature 394:891–894
Manahan-Vaughan D, Braunewell KH (1997) Novelty acquisition is associated with induction of hippocampal long term depression. Proc Natl Acad Sci U S A 96(15):8739–8744
Gholizadeh S, Halder SK, Hampson DR (2015) Expression of fragile X mental retardation protein in neurons and glia in the developing and adult mouse brain. Brain Res 1596:22–30
Bonaccorso CM, Spatuzza M, Di Marco B, Gloria A, Barrancotto G, Cupo A, Musumeci SA, D'Antoni S et al (2015) Fragile X mental retardation (FMRP) interacting proteins exhibit different expression patterns during development. Int J Dev Neurosci 42:15–23
Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT (1997) Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA 94:5401–5404
Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ et al (2001) Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet 98:161–167
Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, Ko HY, Lin GG, Govindarajan A et al (2013) Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc Natl Acad Sci USA 110:5671–5676
Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, Lauterborn JC (2010) Physiological activation of synaptic Rac > PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci 30(33):10977–10984
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y (2004) Rational design and characterization of a rac GTPase-specific small molecular inhibitor. Proc Natl Acad Sci U S A 101(20):7618–7623
Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ (2007) Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem 282(49):35666–35678
Ling Q, Tejada-Simon MV (2015) Statins and the brain: new perspective for old drugs. Prog Neuro-Psychopharmacol Biol Psychiatry 66:80–86
Fatemi SH, Folsom TD, Kneeland RE, Yousef MK, Liesch SB, Thuras PD (2013) Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cereballar vermis and superior frontal cortex. Mol Autism 4:21
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experiments with animals were carried out according to the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH) and approved by the Institutional Animal Care and Use committee (IACUC) at the University of Houston.
Funding
This research has been possible with funding from the FRAXA Research Foundation, the Jerome LeJeune Foundation (France), GEAR-UH grant program, and SGP-UH grant program (M.V.T.S).
Competing Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Martinez, L.A., Tejada-Simon, M.V. Increased Training Intensity Induces Proper Membrane Localization of Actin Remodeling Proteins in the Hippocampus Preventing Cognitive Deficits: Implications for Fragile X Syndrome. Mol Neurobiol 55, 4529–4542 (2018). https://doi.org/10.1007/s12035-017-0666-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0666-4