Abstract
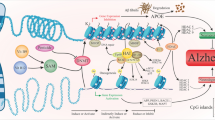
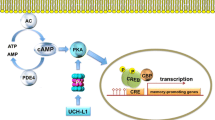
Alzheimer’s disease (AD) is a complex neurodegenerative disorder characterized in the early stages by loss of learning and memory. However, the mechanism underlying these symptoms remains unclear. The best correlation between cognitive decline and pathological changes is in synaptic dysfunction. Histopathological hallmarks of AD are the abnormal aggregation of Aβ and Tau. Evidence suggests that Aβ and Tau oligomers contribute to synaptic loss in AD. Recently, direct links between epigenetic alterations, such as dysfunction in non-coding RNAs (ncRNAs), and synaptic pathologies have emerged, raising interest in exploring the potential roles of ncRNAs in the synaptic deficits in AD. In this paper, we summarize the potential roles of Aβ, Tau, and epigenetic alterations (especially by ncRNAs) in the synaptic dysfunction of AD and discuss the novel findings in this area.


Similar content being viewed by others
References
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368(9533):387–403. doi:10.1016/S0140-6736(06)69113-7
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ et al (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71(5):362–381. doi:10.1097/NEN.0b013e31825018f7
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1(1):a006189. doi:10.1101/cshperspect.a006189
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27(5):457–464. doi:10.1002/ana.410270502
Marksteiner J, Kaufmann WA, Gurka P, Humpel C (2002) Synaptic proteins in Alzheimer’s disease. J Mol Neurosci: MN 18(1–2):53–63. doi:10.1385/JMN:18:1-2:53
Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW Jr, Morris JC (2001) Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56(1):127–129
Brown DF, Risser RC, Bigio EH, Tripp P, Stiegler A, Welch E, Eagan KP, Hladik CL et al (1998) Neocortical synapse density and Braak stage in the Lewy body variant of Alzheimer disease: a comparison with classic Alzheimer disease and normal aging. J Neuropathol Exp Neurol 57(10):955–960
Scheff SW, DeKosky ST, Price DA (1990) Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 11(1):29–37
Mota SI, Ferreira IL, Rego AC (2014) Dysfunctional synapse in Alzheimer’s disease—a focus on NMDA receptors. Neuropharmacology 76 Pt A:16–26. doi:10.1016/j.neuropharm.2013.08.013
Masliah E (1995) Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol 10(2):509–519
Coleman PD, Yao PJ (2003) Synaptic slaughter in Alzheimer’s disease. Neurobiol Aging 24(8):1023–1027
Tu S, Okamoto S, Lipton SA, Xu H (2014) Oligomeric Abeta-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegener 9:48. doi:10.1186/1750-1326-9-48
Pooler AM, Noble W, Hanger DP (2014) A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology 76 Pt A:1–8. doi:10.1016/j.neuropharm.2013.09.018
Guennewig B, Cooper AA (2014) The central role of noncoding RNA in the brain. Int Rev Neurobiol 116:153–194. doi:10.1016/B978-0-12-801105-8.00007-2
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K et al (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325(6106):733–736. doi:10.1038/325733a0
Selkoe DJ (2008) Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res 192(1):106–113. doi:10.1016/j.bbr.2008.02.016
Arendt T (2009) Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol 118(1):167–179. doi:10.1007/s00401-009-0536-x
De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ et al (2009) Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A 106(6):1971–1976. doi:10.1073/pnas.0809158106
Kelly BL, Ferreira A (2006) Beta-amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem 281(38):28079–28089. doi:10.1074/jbc.M605081200
Wang X, Takata T, Bai X, Ou F, Yokono K, Sakurai T (2012) Pyruvate prevents the inhibition of the long-term potentiation induced by amyloid-beta through protein phosphatase 2A inactivation. J Alzheimers Dis : JAD 30(3):665–673. doi:10.3233/JAD-2012-101869
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci Off J Soc Neurosci 27(11):2866–2875. doi:10.1523/JNEUROSCI.4970-06.2007
Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P et al (2011) Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci Off J Soc Neurosci 31(2):700–711. doi:10.1523/JNEUROSCI.4152-10.2011
Figueiredo CP, Clarke JR, Ledo JH, Ribeiro FC, Costa CV, Melo HM, Mota-Sales AP, Saraiva LM et al (2013) Memantine rescues transient cognitive impairment caused by high-molecular-weight abeta oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci Off J Soc Neurosci 33(23):9626–9634. doi:10.1523/JNEUROSCI.0482-13.2013
Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T et al (2013) Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A 110(27):E2518–E2527. doi:10.1073/pnas.1306832110
Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ (2011) Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci Off J Soc Neurosci 31(18):6627–6638. doi:10.1523/JNEUROSCI.0203-11.2011
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D (2009) Soluble oligomers of amyloid beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62(6):788–801. doi:10.1016/j.neuron.2009.05.012
Sinnen BL, Bowen AB, Gibson ES, Kennedy MJ (2016) Local and use-dependent effects of beta-amyloid oligomers on NMDA receptor function revealed by optical Quantal analysis. J Neurosci Off J Soc Neurosci 36(45):11532–11543. doi:10.1523/JNEUROSCI.1603-16.2016
Texido L, Martin-Satue M, Alberdi E, Solsona C, Matute C (2011) Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium 49(3):184–190. doi:10.1016/j.ceca.2011.02.001
Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M et al (2010) Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem 285(10):7619–7632. doi:10.1074/jbc.M109.057182
Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM (2010) Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci Off J Soc Neurosci 30(18):6367–6374. doi:10.1523/JNEUROSCI.0395-10.2010
Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457(7233):1128–1132. doi:10.1038/nature07761
Chung E, Ji Y, Sun Y, Kascsak RJ, Kascsak RB, Mehta PD, Strittmatter SM, Wisniewski T (2010) Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer’s disease model mouse. BMC Neurosci 11:130. doi:10.1186/1471-2202-11-130
Choi BR, Cho WH, Kim J, Lee HJ, Chung C, Jeon WK, Han JS (2014) Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer’s disease. Exp Mol Med 46:e75. doi:10.1038/emm.2013.147
Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L et al (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382(6593):685–691. doi:10.1038/382685a0
Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S et al (2012) A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 122(4):1377–1392. doi:10.1172/JCI58642
Miyamoto T, Kim D, Knox JA, Johnson E, Mucke L (2016) Increasing the receptor tyrosine kinase EphB2 prevents amyloid-beta-induced depletion of cell surface glutamate receptors by a mechanism that requires the PDZ-binding motif of EphB2 and neuronal activity. J Biol Chem 291(4):1719–1734. doi:10.1074/jbc.M115.666529
Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G et al (2011) Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature 469(7328):47–52. doi:10.1038/nature09635
Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA et al (2000) Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106(12):1489–1499. doi:10.1172/JCI10498
Origlia N, Arancio O, Domenici L, Yan SS (2009) MAPK, beta-amyloid and synaptic dysfunction: the role of RAGE. Expert Rev Neurother 9(11):1635–1645. doi:10.1586/ern.09.107
Morroni F, Sita G, Tarozzi A, Rimondini R, Hrelia P (2016) Early effects of Abeta1-42 oligomers injection in mice: involvement of PI3K/Akt/GSK3 and MAPK/ERK1/2 pathways. Behav Brain Res 314:106–115. doi:10.1016/j.bbr.2016.08.002
Wang Y, Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17(1):5–21. doi:10.1038/nrn.2015.1
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12(10):383–388
Cowan CM, Mudher A (2013) Are tau aggregates toxic or protective in tauopathies? Front Neurol 4:114. doi:10.3389/fneur.2013.00114
Fein JA, Sokolow S, Miller CA, Vinters HV, Yang F, Cole GM, Gylys KH (2008) Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol 172(6):1683–1692. doi:10.2353/ajpath.2008.070829
Takahashi RH, Capetillo-Zarate E, Lin MT, Milner TA, Gouras GK (2010) Co-occurrence of Alzheimer’s disease ss-amyloid and tau pathologies at synapses. Neurobiol Aging 31(7):1145–1152. doi:10.1016/j.neurobiolaging.2008.07.021
Castillo-Carranza DL, Guerrero-Munoz MJ, Sengupta U, Hernandez C, Barrett AD, Dineley K, Kayed R (2015) Tau immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer’s disease mouse model. J Neurosci Off J Soc Neurosci 35(12):4857–4868. doi:10.1523/JNEUROSCI.4989-14.2015
Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO et al (2007) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55(5):697–711. doi:10.1016/j.neuron.2007.07.025
Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ (2011) Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A 108(14):5819–5824. doi:10.1073/pnas.1017033108
Zempel H, Thies E, Mandelkow E, Mandelkow EM (2010) Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous tau into dendrites, tau phosphorylation, and destruction of microtubules and spines. J Neurosci Off J Soc Neurosci 30(36):11938–11950. doi:10.1523/JNEUROSCI.2357-10.2010
Zempel H, Mandelkow EM (2012) Linking amyloid-beta and tau: amyloid-beta induced synaptic dysfunction via local wreckage of the neuronal cytoskeleton. Neurodegener Dis 10(1–4):64–72. doi:10.1159/000332816
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC et al (2010) Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142(3):387–397. doi:10.1016/j.cell.2010.06.036
Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E et al (2005) Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci Off J Soc Neurosci 25(42):9694–9703. doi:10.1523/JNEUROSCI.2980-05.2005
Chabrier MA, Cheng D, Castello NA, Green KN, LaFerla FM (2014) Synergistic effects of amyloid-beta and wild-type human tau on dendritic spine loss in a floxed double transgenic model of Alzheimer’s disease. Neurobiol Dis 64:107–117. doi:10.1016/j.nbd.2014.01.007
Tai HC, Wang BY, Serrano-Pozo A, Frosch MP, Spires-Jones TL, Hyman BT (2014) Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer’s disease. Acta Neuropathol Commun 2:146. doi:10.1186/s40478-014-0146-2
Dickstein DL, Brautigam H, Stockton SD Jr, Schmeidler J, Hof PR (2010) Changes in dendritic complexity and spine morphology in transgenic mice expressing human wild-type tau. Brain Struct Funct 214(2–3):161–179. doi:10.1007/s00429-010-0245-1
Polydoro M, Acker CM, Duff K, Castillo PE, Davies P (2009) Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci Off J Soc Neurosci 29(34):10741–10749. doi:10.1523/JNEUROSCI.1065-09.2009
Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L et al (2011) Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic tau mutant. J Neurosci Off J Soc Neurosci 31(7):2511–2525. doi:10.1523/JNEUROSCI.5245-10.2011
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309(5733):476–481. doi:10.1126/science.1113694
Vanderweyde T, Apicco DJ, Youmans-Kidder K, Ash PE, Cook C, Lummertz da Rocha E, Jansen-West K, Frame AA et al (2016) Interaction of tau with the RNA-binding protein TIA1 regulates tau pathophysiology and toxicity. Cell Rep 15(7):1455–1466. doi:10.1016/j.celrep.2016.04.045
Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC (1996) Regulation of the phosphorylation state and microtubule-binding activity of tau by protein phosphatase 2A. Neuron 17(6):1201–1207
Agarwal-Mawal A, Qureshi HY, Cafferty PW, Yuan Z, Han D, Lin R, Paudel HK (2003) 14-3-3 connects glycogen synthase kinase-3 beta to tau within a brain microtubule-associated tau phosphorylation complex. J Biol Chem 278(15):12722–12728. doi:10.1074/jbc.M211491200
Maurin H, Seymour CM, Lechat B, Borghgraef P, Devijver H, Jaworski T, Schmidt MV, Kuegler S et al (2013) Tauopathy differentially affects cell adhesion molecules in mouse brain: Early down-regulation of nectin-3 in stratum lacunosum moleculare. PLoS ONE 8(5):e63589. doi:10.1371/journal.pone.0063589
Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML, Spires-Jones TL, Bacskai BJ et al (2014) Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci U S A 111(1):510–514. doi:10.1073/pnas.1318807111
Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293(5530):711–714. doi:10.1126/science.1062382
Alonso Adel C, Li B, Grundke-Iqbal I, Iqbal K (2006) Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci U S A 103(23):8864–8869. doi:10.1073/pnas.0603214103
Tracy TE, Gan L (2017) Acetylated tau in Alzheimer’s disease: an instigator of synaptic dysfunction underlying memory loss: increased levels of acetylated tau blocks the postsynaptic signaling required for plasticity and promotes memory deficits associated with tauopathy. BioEssays : News Rev Mol Cell Dev Biol. doi:10.1002/bies.201600224
Zempel H, Mandelkow E (2014) Lost after translation: missorting of tau protein and consequences for Alzheimer disease. Trends Neurosci 37(12):721–732. doi:10.1016/j.tins.2014.08.004
King ME, Kan HM, Baas PW, Erisir A, Glabe CG, Bloom GS (2006) Tau-dependent microtubule disassembly initiated by prefibrillar beta-amyloid. J Cell Biol 175(4):541–546. doi:10.1083/jcb.200605187
Pickett EK, Henstridge CM, Allison E, Pitstick R, Pooler A, Wegmann S, Carlson G, Hyman BT et al (2017) Spread of tau down neural circuits precedes synapse and neuronal loss in the rTgTauEC mouse model of early Alzheimer’s disease. Synapse. doi:10.1002/syn.21965
Xie AJ, Liu EJ, Huang HZ, Hu Y, Li K, Lu Y, Wang JZ, Zhu LQ (2016) Cnga2 knockout mice display Alzheimer’s-like behavior abnormities and pathological changes. Mol Neurobiol 53(7):4992–4999. doi:10.1007/s12035-015-9421-x
Li K, Liu FF, He CX, Huang HZ, Xie AJ, Hu F, Liu D, Wang JZ et al (2016) Olfactory deprivation hastens Alzheimer-like pathologies in a human tau-overexpressed mouse model via activation of cdk5. Mol Neurobiol 53(1):391–401. doi:10.1007/s12035-014-9007-z
Gorlovoy P, Larionov S, Pham TT, Neumann H (2009) Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J : Off Publ Fed Am Soc Exp Biol 23(8):2502–2513. doi:10.1096/fj.08-123877
Reitz C, Brayne C, Mayeux R (2011) Epidemiology of Alzheimer disease. Nat Rev Neurol 7(3):137–152. doi:10.1038/nrneurol.2011.2
Holt CE, Schuman EM (2013) The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 80(3):648–657. doi:10.1016/j.neuron.2013.10.036
Smalheiser NR (2014) The RNA-centred view of the synapse: non-coding RNAs and synaptic plasticity. Philos Trans R Soc London Ser B, Biol Sci 369 (1652). doi:10.1098/rstb.2013.0504
Lau P, Bossers K, Janky R, Salta E, Frigerio CS, Barbash S, Rothman R, Sierksma AS et al (2013) Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol Med 5(10):1613–1634. doi:10.1002/emmm.201201974
Hebert SS, De Strooper B (2009) Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci 32(4):199–206. doi:10.1016/j.tins.2008.12.003
Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM et al (2010) Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65(3):373–384. doi:10.1016/j.neuron.2010.01.005
Salta E, Sierksma A, Vanden Eynden E, De Strooper B (2016) miR-132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer’s brain. EMBO Mol Med 8(9):1005–1018. doi:10.15252/emmm.201606520
Shaltiel G, Hanan M, Wolf Y, Barbash S, Kovalev E, Shoham S, Soreq H (2013) Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Struct Funct 218(1):59–72. doi:10.1007/s00429-011-0376-z
Wanet A, Tacheny A, Arnould T, Renard P (2012) miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res 40(11):4742–4753. doi:10.1093/nar/gks151
Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K et al (2008) An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A 105(26):9093–9098. doi:10.1073/pnas.0803072105
Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH (2007) Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci 10(12):1513–1514. doi:10.1038/nn2010
Wibrand K, Pai B, Siripornmongcolchai T, Bittins M, Berentsen B, Ofte ML, Weigel A, Skaftnesmo KO et al (2012) MicroRNA regulation of the synaptic plasticity-related gene arc. PLoS ONE 7(7):e41688. doi:10.1371/journal.pone.0041688
Agostini M, Tucci P, Steinert JR, Shalom-Feuerstein R, Rouleau M, Aberdam D, Forsythe ID, Young KW et al (2011) microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A 108(52):21099–21104. doi:10.1073/pnas.1112063108
Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, Sander C, Tuschl T et al (2009) Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63(6):803–817. doi:10.1016/j.neuron.2009.05.029
Chandrasekar V, Dreyer JL (2009) microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci 42(4):350–362. doi:10.1016/j.mcn.2009.08.009
Xiong YS, Liu FF, Liu D, Huang HZ, Wei N, Tan L, Chen JG, Man HY et al (2015) Opposite effects of two estrogen receptors on tau phosphorylation through disparate effects on the miR-218/PTPA pathway. Aging Cell 14(5):867–877. doi:10.1111/acel.12366
van Spronsen M, van Battum EY, Kuijpers M, Vangoor VR, Rietman ML, Pothof J, Gumy LF, van Ijcken WF et al (2013) Developmental and activity-dependent miRNA expression profiling in primary hippocampal neuron cultures. PLoS ONE 8(10):e74907. doi:10.1371/journal.pone.0074907
Boese AS, Saba R, Campbell K, Majer A, Medina S, Burton L, Booth TF, Chong P et al (2016) MicroRNA abundance is altered in synaptoneurosomes during prion disease. Mol Cell Neurosci 71:13–24. doi:10.1016/j.mcn.2015.12.001
Liu HY, Huang CM, Hung YF, Hsueh YP (2015) The microRNAs Let7c and miR21 are recognized by neuronal toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol 269:202–212. doi:10.1016/j.expneurol.2015.04.011
Shioya M, Obayashi S, Tabunoki H, Arima K, Saito Y, Ishida T, Satoh J (2010) Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol Appl Neurobiol 36(4):320–330. doi:10.1111/j.1365-2990.2010.01076.x
Giusti SA, Vogl AM, Brockmann MM, Vercelli CA, Rein ML, Trumbach D, Wurst W, Cazalla D, Stein V, Deussing JM, Refojo D (2014) MicroRNA-9 controls dendritic development by targeting REST. eLife 3. doi:10.7554/eLife.02755
Chang F, Zhang LH, Xu WP, Jing P, Zhan PY (2014) microRNA-9 attenuates amyloidbeta-induced synaptotoxicity by targeting calcium/calmodulin-dependent protein kinase kinase 2. Mol Med Rep 9(5):1917–1922. doi:10.3892/mmr.2014.2013
Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N (2012) microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci. doi:10.1038/nn.3082
Wei C, Thatcher EJ, Olena AF, Cha DJ, Perdigoto AL, Marshall AF, Carter BD, Broadie K et al (2013) miR-153 regulates SNAP-25, synaptic transmission, and neuronal development. PLoS ONE 8(2):e57080. doi:10.1371/journal.pone.0057080
Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y et al (2010) MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells 28(6):1060–1070. doi:10.1002/stem.431
Gao Y, Su J, Guo W, Polich ED, Magyar DP, Xing Y, Li H, Smrt RD et al (2015) Inhibition of miR-15a promotes BDNF expression and rescues dendritic maturation deficits in MeCP2-deficient neurons. Stem Cells 33(5):1618–1629. doi:10.1002/stem.1950
Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J et al (2008) Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimer’s Dis: JAD 14(1):27–41
Bekris LM, Leverenz JB (2015) The biomarker and therapeutic potential of miRNA in Alzheimer’s disease. Neurodegener Dis Manag 5(1):61–74. doi:10.2217/nmt.14.52
Banzhaf-Strathmann J, Benito E, May S, Arzberger T, Tahirovic S, Kretzschmar H, Fischer A, Edbauer D (2014) MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J 33(15):1667–1680. doi:10.15252/embj.201387576
Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR (2010) Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20(4):492–498. doi:10.1002/hipo.20646
Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6(5):331–341. doi:10.14336/AD.2015.0825
Impey S, Davare M, Lesiak A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR et al (2010) An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci 43(1):146–156. doi:10.1016/j.mcn.2009.10.005
Huang Y, Guo J, Wang Q, Chen Y (2014) MicroRNA-132 silencing decreases the spontaneous recurrent seizures. Int J Clin Exp Med 7(7):1639–1649
Hernandez-Rapp J, Rainone S, Goupil C, Dorval V, Smith PY, Saint-Pierre M, Vallee M, Planel E et al (2016) microRNA-132/212 deficiency enhances Abeta production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci Report 6:30953. doi:10.1038/srep30953
Wang X, Liu P, Zhu H, Xu Y, Ma C, Dai X, Huang L, Liu Y et al (2009) miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Res Bull 80(4–5):268–273. doi:10.1016/j.brainresbull.2009.08.006
Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I et al (2011) microRNA-34c is a novel target to treat dementias. EMBO J 30(20):4299–4308. doi:10.1038/emboj.2011.327
Washietl S, Kellis M, Garber M (2014) Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res 24(4):616–628. doi:10.1101/gr.165035.113
Necsulea A, Soumillon M, Warnefors M, Liechti A, Daish T, Zeller U, Baker JC, Grutzner F et al (2014) The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 505(7485):635–640. doi:10.1038/nature12943
Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, Zhang MQ, Sedel F et al (2010) A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J 29(18):3082–3093. doi:10.1038/emboj.2010.199
Wang H, Iacoangeli A, Popp S, Muslimov IA, Imataka H, Sonenberg N, Lomakin IB, Tiedge H (2002) Dendritic BC1 RNA: functional role in regulation of translation initiation. J Neurosci Off J Soc Neurosci 22(23):10232–10241
Muddashetty R, Khanam T, Kondrashov A, Bundman M, Iacoangeli A, Kremerskothen J, Duning K, Barnekow A et al (2002) Poly(a)-binding protein is associated with neuronal BC1 and BC200 ribonucleoprotein particles. J Mol Biol 321(3):433–445
Mus E, Hof PR, Tiedge H (2007) Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A 104(25):10679–10684. doi:10.1073/pnas.0701532104
Modarresi F, Faghihi MA, Lopez-Toledano MA, Fatemi RP, Magistri M, Brothers SP, van der Brug MP, Wahlestedt C (2012) Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol 30(5):453–459. doi:10.1038/nbt.2158
Peng S, Wuu J, Mufson EJ, Fahnestock M (2005) Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem 93(6):1412–1421. doi:10.1111/j.1471-4159.2005.03135.x
Zhao T, Xu J, Liu L, Bai J, Wang L, Xiao Y, Li X, Zhang L (2015) Computational identification of epigenetically regulated lncRNAs and their associated genes based on integrating genomic data. FEBS Lett 589(4):521–531. doi:10.1016/j.febslet.2015.01.013
Lee DY, Moon J, Lee ST, Jung KH, Park DK, Yoo JS, Sunwoo JS, Byun JI et al (2015) Distinct expression of long non-coding RNAs in an Alzheimer’s disease model. J Alzheimer’s Dis: JAD 45(3):837–849. doi:10.3233/JAD-142919
Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G III et al (2008) Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 14(7):723–730. doi:10.1038/nm1784
Zhao HY, Wu HJ, He JL, Zhuang JH, Liu ZY, Huang LQ, Zhao ZX (2017) Chronic sleep restriction induces cognitive deficits and cortical beta-amyloid deposition in mice via BACE1-antisense activation. CNS Neurosci Ther 23(3):233–240. doi:10.1111/cns.12667
Modarresi F, Faghihi MA, Patel NS, Sahagan BG, Wahlestedt C, Lopez-Toledano MA (2011) Knockdown of BACE1-AS nonprotein-coding transcript modulates beta-amyloid-related hippocampal neurogenesis. Int J Alzheimers Dis 2011:929042. doi:10.4061/2011/929042
Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO (2012) Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 7(2):e30733. doi:10.1371/journal.pone.0030733
Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M et al (2015) Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 58(5):870–885. doi:10.1016/j.molcel.2015.03.027
You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, Akbalik G, Wang M et al (2015) Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 18(4):603–610. doi:10.1038/nn.3975
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388. doi:10.1038/nature11993
Lukiw WJ (2013) Circular RNA (circRNA) in Alzheimer’s disease (AD). Front Genet 4:307. doi:10.3389/fgene.2013.00307
Lonskaya I, Shekoyan AR, Hebron ML, Desforges N, Algarzae NK, Moussa CE (2013) Diminished parkin solubility and co-localization with intraneuronal amyloid-beta are associated with autophagic defects in Alzheimer’s disease. J Alzheimer’s Dis: JAD 33(1):231–247. doi:10.3233/JAD-2012-121141
Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF et al (2009) A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol 11(6):705–716. doi:10.1038/ncb1876
Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, Smalheiser NR (2015) Plasma exosomal miRNAs in persons with and without Alzheimer disease: altered expression and prospects for biomarkers. PLoS ONE 10(10):e0139233. doi:10.1371/journal.pone.0139233
Schroder J, Ansaloni S, Schilling M, Liu T, Radke J, Jaedicke M, Schjeide BM, Mashychev A et al (2014) MicroRNA-138 is a potential regulator of memory performance in humans. Front Hum Neurosci 8:501. doi:10.3389/fnhum.2014.00501
Fuso A, Seminara L, Cavallaro RA, D’Anselmi F, Scarpa S (2005) S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci 28(1):195–204. doi:10.1016/j.mcn.2004.09.007
Chen KL, Wang SS, Yang YY, Yuan RY, Chen RM, Hu CJ (2009) The epigenetic effects of amyloid-beta(1-40) on global DNA and neprilysin genes in murine cerebral endothelial cells. Biochem Biophys Res Commun 378(1):57–61. doi:10.1016/j.bbrc.2008.10.173
Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ et al (2000) Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med 6(2):143–150. doi:10.1038/72237
Rao JS, Keleshian VL, Klein S, Rapoport SI (2012) Epigenetic modifications in frontal cortex from Alzheimer’s disease and bipolar disorder patients. Transl Psychiatry 2:e132. doi:10.1038/tp.2012.55
Fang M, Wang J, Zhang X, Geng Y, Hu Z, Rudd JA, Ling S, Chen W et al (2012) The miR-124 regulates the expression of BACE1/beta-secretase correlated with cell death in Alzheimer’s disease. Toxicol Lett 209(1):94–105. doi:10.1016/j.toxlet.2011.11.032
Conaco C, Otto S, Han JJ, Mandel G (2006) Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A 103(7):2422–2427. doi:10.1073/pnas.0511041103
Kukucka J, Wyllie T, Read J, Mahoney L, Suphioglu C (2013) Human neuronal cells: epigenetic aspects. Biomol concepts 4(4):319–333. doi:10.1515/bmc-2012-0053
Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y et al (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459(7243):55–60. doi:10.1038/nature07925
Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL et al (2010) Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328(5979):753–756. doi:10.1126/science.1186088
Wagner FF, Zhang YL, Fass DM, Joseph N, Gale JP, Weiwer M, McCarren P, Fisher SL et al (2015) Kinetically selective inhibitors of histone deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci 6(1):804–815. doi:10.1039/C4SC02130D
Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM et al (2012) An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483(7388):222–226. doi:10.1038/nature10849
Herrup K, Li J, Chen J (2013) The role of ATM and DNA damage in neurons: upstream and downstream connections. DNA repair 12(8):600–604. doi:10.1016/j.dnarep.2013.04.012
Sando R III, Gounko N, Pieraut S, Liao L, Yates J III, Maximov A (2012) HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell 151(4):821–834. doi:10.1016/j.cell.2012.09.037
Quintas A, de Solis AJ, Diez-Guerra FJ, Carrascosa JM, Bogonez E (2012) Age-associated decrease of SIRT1 expression in rat hippocampus: prevention by late onset caloric restriction. Exp Gerontol 47(2):198–201. doi:10.1016/j.exger.2011.11.010
Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D et al (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466(7310):1105–1109. doi:10.1038/nature09271
Acknowledgments
This study was supported partially by the National Natural Science Foundation of China (31571039, 91632114); the Top-Notch Young Talents Program of China of 2014; the Program of Outstanding Youth of Hubei Province, China (2014CFA017); and the Academic Frontier Youth Team of Huazhong University of Science and Technology to Dr. Ling-Qiang Zhu.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Li, K., Wei, Q., Liu, FF. et al. Synaptic Dysfunction in Alzheimer’s Disease: Aβ, Tau, and Epigenetic Alterations. Mol Neurobiol 55, 3021–3032 (2018). https://doi.org/10.1007/s12035-017-0533-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0533-3