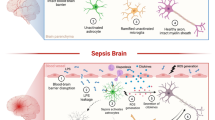
Abstract
Severe systemic inflammation has strong effects on brain functions, promoting permanent neurocognitive dysfunction and high mortality rates. Additionally, hippocampal damage seems to be directly involved in this process and astrocytes play an important role in neuroinflammation and in the neuroimmune response. However, the contribution of the astrocytes to the pathology of acute brain dysfunction is not well understood. Recently, our group established a protocol for obtaining astrocyte cultures from mature brain to allow the characterization of these cells and their functions under pathologic conditions. The present study was designed to characterize astrocyte function after acute systemic inflammation induced by cecal ligation and perforation (CLP). Hippocampal astrocyte cultures from CLP animals presented increased levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-18, and cyclooxygenase-2 and decreased levels of IL-10. This proinflammatory profile was accompanied by an increase in Toll-like receptor (TLR)2 mRNA expression levels and no change either in TLR4 or in vascular endothelial growth factor (VEGF) gene expression. These alterations were associated with increased expressions of p21, nuclear factor kappa B (NFκB), and inducible nitric oxide synthase (iNOS) in astrocytes from CLP animals. The same parameters were also evaluated in whole hippocampal tissue, but differences in this profile were found compared to hippocampal astrocyte cultures from CLP, reflecting an interaction between other central nervous system cell types, which may mask specific astrocytic changes. These results improve our understanding of the mechanisms by which astrocytes react against systemic inflammation, and suggest these cells to be potential targets for therapeutic modulation.






Similar content being viewed by others
Change history
26 May 2020
The givenname ���Paola��� of the author Paola Haack Amaral Roppa (Roppa, P.H.A.) should be corrected to read Ricardo Haack Amaral Roppa (Roppa, R.H.A.) as presented above.
References
Fu HQ, Yang T, Xiao W, Fan L, Wu Y, Terrando N, Wang TL (2014) Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS One 9(8):e106331. doi:10.1371/journal.pone.0106331
Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M (2010) Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 107(47):20518–20522. doi:10.1073/pnas.1014557107
Michels M, Steckert AV, Quevedo J, Barichello T, Dal-Pizzol F (2015) Mechanisms of long-term cognitive dysfunction of sepsis: from blood-borne leukocytes to glial cells. Intensive care medicine experimental 3(1):30. doi:10.1186/s40635-015-0066-x
Esper AM, Martin GS (2009) Extending international sepsis epidemiology: the impact of organ dysfunction. Critical care (London, England) 13(1):120. doi:10.1186/cc7704
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 311(13):1308–1316. doi:10.1001/jama.2014.2637
Cotena S, Piazza O (2012) Sepsis-associated encephalopathy. Translational medicine @ UniSa 2:20–27
Mazeraud A, Pascal Q, Verdonk F, Heming N, Chretien F, Sharshar T (2016) Neuroanatomy and physiology of brain dysfunction in sepsis. Clin Chest Med 37(2):333–345. doi:10.1016/j.ccm.2016.01.013
Danielski LG, Giustina AD, Badawy M, Barichello T, Quevedo J, Dal-Pizzol F, Petronilho F (2017) Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol. doi:10.1007/s12035-016-0356-7
Tsujimoto H, Ono S, Efron PA, Scumpia PO, Moldawer LL, Mochizuki H (2008) Role of Toll-like receptors in the development of sepsis. Shock (Augusta, Ga) 29(3):315–321. doi:10.1097/SHK.0b013e318157ee55
Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59(2):242–255. doi:10.1002/glia.21094
Lloberas J, Celada A (2009) p21(waf1/CIP1), a CDK inhibitor and a negative feedback system that controls macrophage activation. Eur J Immunol 39(3):691–694. doi:10.1002/eji.200939262
Yang QH, Liu DW, Wang XT, Yang RL, Shi Y, Long Y, Liu HZ, He HW et al (2011) G1 cell cycle arrest signaling in hepatic injury after intraperitoneal sepsis in rats. Inflammation research: official journal of the European Histamine Research Society [et al] 60(8):783–789. doi:10.1007/s00011-011-0334-5
Balomenos D, Martin-Caballero J, Garcia MI, Prieto I, Flores JM, Serrano M, Martinez AC (2000) The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat Med 6(2):171–176. doi:10.1038/72272
Rackov G, Hernandez-Jimenez E, Shokri R, Carmona-Rodriguez L, Manes S, Alvarez-Mon M, Lopez-Collazo E, Martinez AC et al (2016) p21 mediates macrophage reprogramming through regulation of p50-p50 NF-kappaB and IFN-beta. J Clin Invest 126(8):3089–3103. doi:10.1172/jci83404
Chapouly C, Tadesse Argaw A, Horng S, Castro K, Zhang J, Asp L, Loo H, Laitman BM et al (2015) Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain: a journal of neurology 138(Pt 6):1548–1567. doi:10.1093/brain/awv077
Mayo L, Quintana FJ, Weiner HL (2012) The innate immune system in demyelinating disease. Immunol Rev 248(1):170–187. doi:10.1111/j.1600-065X.2012.01135.x
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2016) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol. doi:10.1007/s12035-016-9880-8
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS One 8:E60282. doi:10.1371/journal.pone.0060282
Zimmer ER, Parent MJ, Souza DG, Leuzy A, Lecrux C, Kim HI, Gauthier S, Pellerin L et al (2017) [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosc. doi:10.1038/nn.4492
Petronilho F, Perico SR, Vuolo F, Mina F, Constantino L, Comim CM, Quevedo J, Souza DO et al (2012) Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun 26(6):904–910. doi:10.1016/j.bbi.2012.03.007
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods (San Diego, Calif) 25(4):402–408. doi:10.1006/meth.2001.1262
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150(1):76–85. doi:10.1016/0003-2697(85)90442-7
Widmann CN, Heneka MT (2014) Long-term cerebral consequences of sepsis. Lancet Neurol 13(6):630–636. doi:10.1016/s1474-4422(14)70017-1
Dejager L, Pinheiro I, Dejonckheere E, Libert C (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19(4):198–208. doi:10.1016/j.tim.2011.01.001
Neves FS, Marques PT, Barros-Aragao F, Nunes JB, Venancio AM, Cozachenco D, Frozza RL, Passos GF et al (2016) Brain-defective insulin signaling is associated to late cognitive impairment in post-septic mice. Mol Neurobiol. doi:10.1007/s12035-016-0307-3
Comim CM, Freiberger V, Ventura L, Mina F, Ferreira GK, Michels M, Generoso JS, Streck EL et al (2017) Inhibition of indoleamine 2,3-dioxygenase 1/2 prevented cognitive impairment and energetic metabolism changes in the hippocampus of adult rats subjected to polymicrobial sepsis. J Neuroimmunol 305:167–171. doi:10.1016/j.jneuroim.2017.02.001
Srinivasan K, Friedman BA, Larson JL, Lauffer BE, Goldstein LD, Appling LL, Borneo J, Poon C et al (2016) Untangling the brain’s neuroinflammatory and neurodegenerative transcriptional responses. Nat Commun 7:11295. doi:10.1038/ncomms11295
Souza DG, Bellaver B, Bobermin LD, Souza DO, Quincozes-Santos A (2016) Anti-aging effects of guanosine in glial cells. Purinergic Signal. doi:10.1007/s11302-016-9533-4
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and deceases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol in Vitro 28:479–484. doi:10.1016/j.tiv.2014.01.006
Dal-Pizzol F, Rojas HA, dos Santos EM, Vuolo F, Constantino L, Feier G, Pasquali M, Comim CM et al (2013) Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol 48(1):62–70. doi:10.1007/s12035-013-8433-7
Comim CM, Vilela MC, Constantino LS, Petronilho F, Vuolo F, Lacerda-Queiroz N, Rodrigues DH, da Rocha JL et al (2011) Traffic of leukocytes and cytokine up-regulation in the central nervous system in sepsis. Intensive Care Med 37(4):711–718. doi:10.1007/s00134-011-2151-2
Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ et al (2012) Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122(7):2454–2468. doi:10.1172/jci60842
Wang X, Kang K, Wang S, Yao J, Zhang X (2016) Focal cerebral ischemic tolerance and change in blood-brain barrier permeability after repetitive pure oxygen exposure preconditioning in a rodent model. J Neurosurg 125(4):943–952. doi:10.3171/2015.7.jns142220
Ma Y, Zechariah A, Qu Y, Hermann DM (2012) Effects of vascular endothelial growth factor in ischemic stroke. J Neurosci Res 90(10):1873–1882. doi:10.1002/jnr.23088
Hanke ML, Kielian T (2011) Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clinical science (London, England: 1979) 121(9):367–387. doi:10.1042/cs20110164
Brightbill HD, Modlin RL (2000) Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology 101(1):1–10. doi:10.1046/j.1365-2567.2000.00093.x
Hanzelmann D, Joo HS, Franz-Wachtel M, Hertlein T, Stevanovic S, Macek B, Wolz C, Gotz F et al (2016) Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat Commun 7:12304. doi:10.1038/ncomms12304
Laflamme N, Soucy G, Rivest S (2001) Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J Neurochem 79(3):648–657. doi:10.1046/j.1471-4159.2001.00603.x
Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, Chierakul W, Leendertse M et al (2007) Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis). PLoS Med 4(7):e248. doi:10.1371/journal.pmed.0040248
Kielian T (2006) Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res 83(5):711–730. doi:10.1002/jnr.20767
Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD (2005) Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49(3):360–374. doi:10.1002/glia.20117
Troutman TD, Bazan JF, Pasare C (2012) Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell cycle (Georgetown, Tex) 11(19):3559–3567. doi:10.4161/cc.21572
Marinelli C, Di Liddo R, Facci L, Bertalot T, Conconi MT, Zusso M, Skaper SD, Giusti P (2015) Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes. J Neuroinflammation 12:244. doi:10.1186/s12974-015-0458-6
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal. doi:10.1089/ars.2010.3216
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41(2–3):242–247. doi:10.1007/s12035-010-8105-9
Aruoma OI, Grootveld M, Bahorun T (2006) Free radicals in biology and medicine: from inflammation to biotechnology. BioFactors (Oxford, England) 27(1–4):1–3. doi:10.1002/biof.5520270101
Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S et al (1994) NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol 153(5):2052–2063
Dranse HJ, Muruganandan S, Fawcett JP, Sinal CJ (2016) Adipocyte-secreted chemerin is processed to a variety of isoforms and influences MMP3 and chemokine secretion through an NFkB-dependent mechanism. Mol Cell Endocrinol 436:114–129. doi:10.1016/j.mce.2016.07.017
Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, Ruderman EM, Samways DS et al (2009) The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol 39(3):820–825. doi:10.1002/eji.200838683
Montes M, Lund AH (2016) Emerging roles of lncRNAs in senescence. FEBS J 283(13):2414–2426. doi:10.1111/febs.13679
Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS, Chesnokova V (2015) Chronic intestinal inflammation alters hippocampal neurogenesis. J Neuroinflammation 12:65. doi:10.1186/s12974-015-0281-0
Tusell JM, Saura J, Serratosa J (2005) Absence of the cell cycle inhibitor p21Cip1 reduces LPS-induced NO release and activation of the transcription factor NF-kappaB in mixed glial cultures. Glia 49(1):52–58. doi:10.1002/glia.20095
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Federal University of Rio Grande do Sul (UFRGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal experiments were performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior’s recommendations for animal care. The experimental protocols were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number 29180).
Conflict of Interest
The authors declare there are no conflicts of interest.
Electronic supplementary material
Supplementary Fig. 1
Inflammatory profile in the whole hippocampus. (A) TNF-α, (B) IL-1β and (G) COX-2 mRNA expression levels. (C) TNF-α, (D) IL-1β, (E) IL-6, (F) IL-18 and MCP-1 levels. Data represent the means + S.E.M. of groups (n= 6-8). *P <0.05, **P <0.01 and ***P <0.001 (t test), compared to the sham group. (TIFF 2874 kb)
Supplementary Fig. 2
CLP promoted increases in mRNA expression levels of (A) NFκB and (B) iNOS in hippocampal tissue. Data represent the means + S.E.M. of groups (n= 6). **P <0.01 (t test), compared to the sham group. (TIFF 1300 kb)
Rights and permissions
About this article
Cite this article
Bellaver, B., dos Santos, J.P., Leffa, D.T. et al. Systemic Inflammation as a Driver of Brain Injury: the Astrocyte as an Emerging Player. Mol Neurobiol 55, 2685–2695 (2018). https://doi.org/10.1007/s12035-017-0526-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0526-2