Abstract
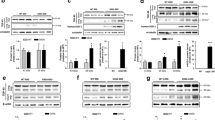
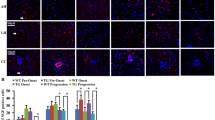
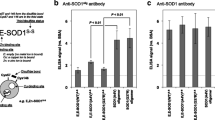
The known proteins only explained the partial pathogenesis of amyotrophic lateral sclerosis (ALS). Therefore, this study aimed to search the novel proteins possibly involved in ALS. In this study, we analyzed the expression and distribution of the candidate protein arylsulfatase B (ARSB) in the different segments, anatomic regions, and neural cells of spinal cord at the different stages of the wild-type and [Cu/Zn] superoxide dismutase 1 (SOD1) G93A transgenic mice using the fluorescent immunohistochemistry and the western blot. The results revealed that the ARSB was extensively expressed and distributed in the entire spinal cord; the expression and distribution of ARSB was significantly different in the different regions of spinal cord, the anterior horn of gray matter (AHGM) was significantly more than that in the posterior horn of gray matter (PHGM) and significantly more than that in the central canal, and ARSB was mainly distributed in the microglia and neuron cells in the wild-type mice. The expression of ARSB significantly increased in other anatomic regions besides the thoracic PHGM, significantly decreased at the progression stage, occurred in the redistribution from the AHGM and the PHGM to the central canal at the onset and progression stages, and no any alteration of ARSB expression and distribution occurred between the different neural cells in the SOD1 G93A mice compared with the wild-type mice. The increase of ARSB expression and distribution followed with the increased of neuron death. Our data suggested that the abnormal expression and distribution of ARSB were closely associated with the neuron death in the SOD1 G93A transgenic mice.













Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- ARSB:
-
Arylsulfatase B
- SOD1:
-
[Cu/Zn] superoxide dismutase 1
- GFAP:
-
Glial fibrillary acidic protein
- NeuN:
-
Neuronal nuclear antigen
- Fox3:
-
RNA binding protein fox-1 homolog 3
- IBA-1:
-
Ionized calcium binding adapter molecule-1
- DAPI:
-
4′,6-diamidino-2-phenylindole
- Hm:
-
Human
References
Riva N, Agosta F, Lunetta C et al (2016) Recent advances in amyotrophic lateral sclerosis. J Neurol 263:1241–1254. doi:10.1007/s00415-016-8091-6
Okado-Matsumoto A, Fridovich I (2002) Amyotrophic lateral sclerosis: a proposed mechanism. Proc Natl Acad Sci U S A 99:9010–9014. doi:10.1073/pnas.132260399
Kiernan MC, Vucic S, Cheah BC et al (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955. doi:10.1016/S0140-6736(10)61156-7
Ludolph AC, Brettschneider J, Weishaupt JH (2012) Amyotrophic lateral sclerosis. Curr Opin Neurol 25:530–535. doi:10.1097/WCO.0b013e328356d328
Yamashita S, Ando Y (2015) Genotype-phenotype relationship in hereditary amyotrophic lateral sclerosis. Transl Neurodegener 4:13. doi:10.1186/s40035-015-0036-y
Zarei S, Carr K, Reiley L et al (2015) A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int 6:171. doi:10.4103/2152-7806.169561
Trojsi F, Monsurrò MR, Tedeschi G (2013) Exposure to environmental toxicants and pathogenesis of amyotrophic lateral sclerosis: state of the art and research perspectives. Int J Mol Sci 14:15286–15311. doi:10.3390/ijms140815286
Ingre C, Roos PM, Piehl F et al (2015) Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol 7:181–193. doi:10.2147/CLEP.S37505
Oskarsson B, Horton DK, Mitsumoto H (2015) Potential environmental factors in amyotrophic lateral sclerosis. Neurol Clin 33:877–888. doi:10.1016/j.ncl.2015.07.009
Kaur SJ, McKeown SR, Rashid S (2016) Mutant SOD1 mediated pathogenesis of amyotrophic lateral sclerosis. Gene 577:109–118. doi:10.1016/j.gene.2015.11.049
Ratti A, Buratti E (2016) Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem 138(Suppl 1):95–111. doi:10.1111/jnc.13625
Kishikawa H, Wu D, Hu GF (2008) Targeting angiogenin in therapy of amyotrophic lateral sclerosis. Expert Opin Ther Targets 12:1229–1242. doi:10.1517/14728222.12.10.1229
Maruyama H, Kawakami H (2013) Optineurin and amyotrophic lateral sclerosis. Geriatr Gerontol Int 13:528–532. doi:10.1111/ggi.12022
Le Ber I (2015) Frontotemporal lobar dementia and amyotrophic lateral sclerosis associated with c9orf72 expansion. Rev Neurol (Paris) 171:475–481. doi:10.1016/j.neurol.2015.04.004
Finsterer J, Burgunder JM (2014) Recent progress in the genetics of motor neuron disease. Eur J Med Genet 57:103–112. doi:10.1016/j.ejmg.2014.01.002
King AE, Woodhouse A, Kirkcaldie MT, Vickers JC (2016) Excitotoxicity in ALS: overstimulation or overreaction? Exp Neurol 275(Pt 1):162–171. doi:10.1016/j.expneurol.2015.09.019
Cozzolino M, Rossi S, Mirra A, Carrì MT (2015) Mitochondrial dynamism and the pathogenesis of amyotrophic lateral sclerosis. Front Cell Neurosci 9:31. doi:10.3389/fncel.2015.00031
Magrané J, Sahawneh MA, Przedborski S et al (2012) Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. J Neurosci 32:229–242. doi:10.1523/JNEUROSCI.1233-11.2012
D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H (2013) Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med 65:509–527. doi:10.1016/j.freeradbiomed.2013.06.029
Ferraiuolo L, Kirby J, Grierson AJ et al (2011) Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Na Rev Neurol 7:616–630. doi:10.1038/nrneurol.2011.152
Turner MR, Hardiman O, Benatar M et al (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12:310–322. doi:10.1016/S1474-4422(13) 70036-X
Leblond CS, Kaneb HM, Dion PA, Rouleau GA (2014) Dissection of genetic factors associated with amyotrophic lateral sclerosis. Exp Neurol 262 Pt B:91–101. doi:10.1016/j.expneurol.2014.04.013
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17:17–23. doi:10.1038/nn.3584
Pasquali L, Lenzi P, Biagioni F et al (2014) Cell to cell spreading of misfolded proteins as a therapeutic target in motor neuron disease. Curr Med Chem 21:3508–3534. doi:10.2174/0929867321666140601161534
Ruegsegger C, Saxena S (2016) Proteostasis impairment in ALS. Brain Res 1648(Pt B):571–579. doi:10.1016/j.brainres.2016.03.032
Litjens T, Hopwood JJ (2001) Mucopolysaccharidosis type VI: structural and clinical implications of mutations in N-acetylgalactosamine-4-sulfatase. Hum Mutat 18:282–295. doi:10.1002/humu.1190
Valayannopoulos V, Nicely H, Harmatz P, Turbeville S (2010) Mucopolysaccharidosis VI. Orphanet J Rare Dis 5:5. doi:10.1186/1750-1172-5-5
Zhang X, Bhattacharyya S, Kusumo H et al (2014) Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: dysregulation by ethanol. Glia 62:259–271. doi:10.1002/glia.22604
Yoo M, Khaled M, Gibbs KM et al (2013) Arylsulfatase B improves locomotor function after mouse spinal cord injury. PLoS One 8:e57415. doi:10.1371/journal.pone.0057415
Freemon FR, Quick DT (1986) Aryl sulfatase in vitamin E deficiency and in amyotrophic lateral sclerosis. Neurology 18:310
Yates CM, Wilson H, Davidson D (1973) Lysosomal enzymes in motor neurone disease and multiple sclerosis. Clin Chim Acta 47:397–402
Gurney ME, Pu H, Chiu AY et al (1994) Motor neurondegeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1775
Zhou Y, Lu Y, Fang X et al (2015) An astrocyte regenerative response from vimentin-containing cells in the spinal cord of amyotrophic lateral sclerosis’s disease-like transgenic (G93A SOD1) mice. Neurodegener Dis 15:1–12. doi:10.1159/000369466
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377–391
Raineteau O, Schwab ME (2001) Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2:263–273. doi:10.1038/35067570
Fehlings MG (2008) Repair of the chronically injured human spinal cord. J Neurosurg Spine 8:205–206 . doi:10.3171/SPI/2008/8/3/205discussion 206-207
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294–301. doi:10.1016/j.expneurol.2007.05.014
McKeon RJ, Jurynec MJ, Buck CR (1999) The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci 19:10778–10788
Asher RA, Morgenstern DA, Fidler PS et al (2000) Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci 20:2427–2438
Jones LL, Margolis RU, Tuszynski MH (2003) The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol 182:399–411. doi:10.1016/S0014-4886(03)00087-6
Brittis PA, Canning DR, Silver J (1992) Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science 255:733–736. doi:10.1126/science.1738848
Silver J (1994) Inhibitory molecules in development and regeneration. J Neurol 242:S22–S24. doi:10.1007/BF00939236
Wilson MT, Snow DM (2000) Chondroitin sulfate proteoglycan expression pattern in hippocampal development: potential regulation of axon tract formation. J Comp Neurol 424:532–546. doi:10.1002/1096-9861(20000828)424:3<532::AID-CNE10>3.0.CO;2-Z
Cavalcante LA, Garcia-Abreu J, Mendes FA et al (2003) Sulfated proteoglycans as modulators of neuronal migration and axonal decussation in the developing midbrain. Braz J Med Biol Res 36:993–1002. doi:10.1590/S0100-879X2003000800005
Pizzorusso T, Medini P, Berardi N et al (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251. doi:10.1126/science.1072699
Deepa SS, Carulli D, Galtrey C et al (2006) Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem 281:17789–17800. doi:10.1074/jbc.M600544200
Carulli D, Rhodes KE, Awcett JW (2007) Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol 501:83–94. doi:10.1002/cne.21231
Bovolenta P, Wandosell F, Nieto-Sampedro M (1991) Neurite outgrowth over resting and reactive astrocytes. Restor Neurol Neurosci 2:221–228. doi:10.3233/RNN-1991-245609
Fitch MT, Doller C, Combs CK et al (1999) Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 19:8182–8198
Morgenstern DA, Asher RA, Fawcett JW (2002) Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res 137:313–332. doi:10.1016/S0079-6123(02)37024-9
Wang H, Katagiri Y, McCann TE et al (2008) Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci 121:3083–3091. doi:10.1242/jcs.032649
Properzi F, Carulli D, Asher RA et al (2005) Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci 21:378–390. doi:10.1111/j.1460-9568.2005.03876.x
Butterfield KC, Caplan M, Panitch A (2010) Identification and sequence composition characterization of chondroitin sulfate-binding peptides through peptide array screening. Biochemistry 49:1549–1555. doi:10.1021/bi9021044
Butterfield KC, Conovaloff A, Caplan M, Panitch A (2010) Chondroitin sulfatebinding peptides block chondroitin 6-sulfate inhibition of cortical neurite growth. Neurosci Lett 478:82–87. doi:10.1016/j.neulet.2010.04.070
Gilbert RJ, McKeon RJ, Darr A et al (2005) CS-4, 6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci 29:545–558. doi:10.1016/j.mcn.2005.04.006
Wasserman SI, Austen KF (1976) Arylsulfatase B of human lung. Isolation, characterization, and interaction with slow-reacting substance of anaphylaxis. J Clin Invest 57:738–744. doi:10.1172/JCI108332
Harmatz P, Ketteridge D, Giugliani R et al (2005) Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics 115:e681–e689. doi:10.1542/peds.2004-1023
Harmatz P, Whitley CB, Waber L et al (2004) Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). J Pediatr 144:574–580. doi:10.1016/j.jpeds.2004.03.018
Muñoz-Rojas MV, Horovitz DD, Jardim LB et al (2010) Intrathecal administration of recombinant human N-acetylgalactosamine 4-sulfatase to a MPS VI patient with pachymeningitis cervicalis. Mol Genet Metab 99:346–350. doi:10.1016/j.ymgme.2009.11.008
Mitsunaga-Nakatsubo K, Kusunoki S, Kawakami H et al (2009) Cell-surface arylsulfatase A and B on sinusoidal endothelial cells, hepatocytes, and Kupffer cells in mammalian livers. Med Mol Morphol 42:63–69. doi:10.1007/s00795-009-0447-x
Acknowledgements
We are grateful to the National Natural Science Foundation of China (30560042, 81160161, 81360198); Education Department of Jiangxi Province (GJJ13198); and Jiangxi provincial department of science and technology ([2014]-47, 20142BBG70062) for supporting this work.
Author Contributions
R. X. and J. Z. conceived and designed the experiments and wrote the manuscript. J. Z., H. L., L. Z., W. G., C. T., and J. L. conducted the experiments and analyzed the data. X. Z. contributed some materials. J. Z., H. L., L. Z., W. G., and C. T. are the first authors and they contributed equally to the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Funding
The work was funded by the National Natural Science Foundation of China (30560042, 81160161, 81360198); Education Department of Jiangxi Province (GJJ13198); and Jiangxi Provincial Department of Science and Technology ([2014]-47, 20142BBG70062).
Additional information
Jie Zhang, Huiting Liang, and Lei Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, J., Liang, H., Zhu, L. et al. Expression and Distribution of Arylsulfatase B are Closely Associated with Neuron Death in SOD1 G93A Transgenic Mice. Mol Neurobiol 55, 1323–1337 (2018). https://doi.org/10.1007/s12035-017-0406-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0406-9