Abstract
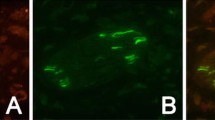
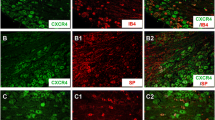
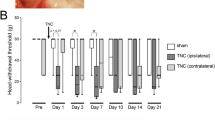
It has been proposed that after nerve injury or tissue inflammation, fractalkine (CX3CL1) released from dorsal root ganglion neurons acts on satellite glial cells (SGCs) through CX3C receptor 1 (CX3CR1) to induce neuroplastic changes. The existence and importance of fractalkine/CX3CR1 signaling in the trigeminal ganglia has not yet been clarified. This study investigated (1) whether trigeminal ganglion neurons that innervate temporalis muscle and their associated SGCs contain fractalkine and/or express CX3CR1, (2) if intraganglionic injection of fractalkine increases the mechanical sensitivity of temporalis muscle afferent fibers, (3) whether complete Freund’s adjuvant (CFA)-induced inflammation of the temporalis muscle alters the expression of fractalkine or its receptor in the trigeminal ganglion, and (4) if intraganglionic administration of CX3CR1 antibodies alters afferent mechanical sensitivity. Immunohistochemistry and in vivo electrophysiological recordings in male and female rats were used to address these questions. It was found that ∼50 % of temporalis ganglion neurons and ∼25 % of their associated SGCs express CX3CR1, while only neurons expressed fractalkine. Temporalis muscle inflammation increased the expression of fractalkine, but only in male rats. Intraganglionic injection of fractalkine (25 g/ml; 3 μl) induced prolonged afferent mechanical sensitization. Intraganglionic injection of CX3CR1 antibody increased afferent mechanical threshold, but this effect was greater in controls than in rats with CFA-induced muscle inflammation. These findings raise the possibility that basal fractalkine signalling within the trigeminal ganglion plays an important role in mechanical sensitivity of masticatory muscle sensory afferent fibers and that inhibition of CX3CR1 signaling within the trigeminal ganglia may induce analgesia through a peripheral mechanism.






Similar content being viewed by others
References
Nomenclature IWSoC (2003) Chemokine/chemokine receptor nomenclature. Cytokine 21(1):48–9
Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA (2009) Chemokines and pain mechanisms. Brain Res Rev 60(1):125–34. doi:10.1016/j.brainresrev.2008.12.002
Guillot X, Semerano L, Decker P, Falgarone G, Boissier MC (2012) Pain and immunity. Joint Bone Spine 79(3):228–36. doi:10.1016/j.jbspin.2011.10.008
Stievano L, Piovan E, Amadori A (2004) C and CX3C chemokines: cell sources and physiopathological implications. Crit Rev Immunol 24(3):205–28
White GE, Greaves DR (2009) Fractalkine: one chemokine, many functions. Blood 113(4):767–8. doi:10.1182/blood-2008-11-189860
Clark AK, Malcangio M (2014) Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci 8:121. doi:10.3389/fncel.2014.00121
Milligan ED, Sloane EM, Watkins LR (2008) Glia in pathological pain: a role for fractalkine. J Neuroimmunol 198(1-2):113–20. doi:10.1016/j.jneuroim.2008.04.011
Souza GR, Talbot J, Lotufo CM, Cunha FQ, Cunha TM, Ferreira SH (2013) Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci U S A 110(27):11193–8. doi:10.1073/pnas.1307445110
Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC (2004) Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci 20(5):1150–60. doi:10.1111/j.1460-9568.2004.03593.x
Zhu W, Acosta C, MacNeil B, Cortes C, Intrater H, Gong Y, Namaka M (2013) Elevated expression of fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) in the dorsal root ganglia and spinal cord in experimental autoimmune encephalomyelitis: implications in multiple sclerosis-induced neuropathic pain. Biomed Res Int 2013:480702. doi:10.1155/2013/480702
Cairns BE, Laursen JC, Dong XD, Gazerani P (2014) Intraganglionic injection of a nitric oxide donator induces afferent mechanical sensitization that is attenuated by palmitoylethanolamide. Cephalalgia 34(9):686–94. doi:10.1177/0333102414521510
Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE (2007) Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience 146(2):822–32. doi:10.1016/j.neuroscience.2007.01.051
Hakim AW, Dong XD, Svensson P, Kumar U, Cairns BE (2009) TNFalpha mechanically sensitizes masseter muscle afferent fibers of male rats. J Neurophysiol 102(3):1551–9. doi:10.1152/jn.00326.2009
Sung D, Dong X, Ernberg M, Kumar U, Cairns BE (2008) Serotonin (5-HT) excites rat masticatory muscle afferent fibers through activation of peripheral 5-HT3 receptors. Pain 134(1-2):41–50. doi:10.1016/j.pain.2007.03.034
Svensson P, Wang MW, Dong XD, Kumar U, Cairns BE (2010) Human nerve growth factor sensitizes masseter muscle nociceptors in female rats. Pain 148(3):473–80. doi:10.1016/j.pain.2009.12.009
Wang MW, Kumar U, Dong XD, Cairns BE (2012) Expression of NMDA and oestrogen receptors by trigeminal ganglion neurons that innervate the rat temporalis muscle. Chin J Dent Res 15(2):89–97
Wong H, Kang I, Dong XD, Christidis N, Ernberg M, Svensson P, Cairns BE (2014) NGF-induced mechanical sensitization of the masseter muscle is mediated through peripheral NMDA receptors. Neuroscience 269:232–44. doi:10.1016/j.neuroscience.2014.03.054
Cairns BE, Gambarota G, Svensson P, Arendt-Nielsen L, Berde CB (2002) Glutamate-induced sensitization of rat masseter muscle fibers. Neuroscience 109(2):389–99
Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P (2001) Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 86(2):782–91
Dong XD, Mann MK, Sessle BJ, Arendt-Nielsen L, Svensson P, Cairns BE (2006) Sensitivity of rat temporalis muscle afferent fibers to peripheral N-methyl-D-aspartate receptor activation. Neuroscience 141(2):939–45. doi:10.1016/j.neuroscience.2006.04.024
Andersen S, Petersen MW, Svendsen AS, Gazerani P (2015) Pressure pain thresholds assessed over temporalis, masseter, and frontalis muscles in healthy individuals, patients with tension-type headache, and those with migraine—a systematic review. Pain 156(8):1409–23. doi:10.1097/j.pain.0000000000000219
Cairns BE (2010) Pathophysiology of TMD pain—basic mechanisms and their implications for pharmacotherapy. J Oral Rehabil 37(6):391–410. doi:10.1111/j.1365-2842.2010.02074.x
Huang ZZ, Li D, Liu CC, Cui Y, Zhu HQ, Zhang WW, Li YY, Xin WJ (2014) CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun 40:155–65. doi:10.1016/j.bbi.2014.03.014
Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001) Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 21(14):5027–35
Oh SB, Endoh T, Simen AA, Ren D, Miller RJ (2002) Regulation of calcium currents by chemokines and their receptors. J Neuroimmunol 123(1-2):66–75
Laursen JC, Cairns BE, Dong XD, Kumar U, Somvanshi RK, Arendt-Nielsen L, Gazerani P (2014) Glutamate dysregulation in the trigeminal ganglion: a novel mechanism for peripheral sensitization of the craniofacial region. Neuroscience 256:23–35. doi:10.1016/j.neuroscience.2013.10.009
Puil E, Spigelman I (1988) Electrophysiological responses of trigeminal root ganglion neurons in vitro. Neuroscience 24(2):635–46
Lauro C, Catalano M, Trettel F, Limatola C (2015) Fractalkine in the nervous system: neuroprotective or neurotoxic molecule? Ann N Y Acad Sci 1351(1):141–8. doi:10.1111/nyas.12805
Sheridan GK, Murphy KJ (2013) Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol 3(12):130181. doi:10.1098/rsob.130181
Deiva K, Geeraerts T, Salim H, Leclerc P, Hery C, Hugel B, Freyssinet JM, Tardieu M (2004) Fractalkine reduces N-methyl-d-aspartate-induced calcium flux and apoptosis in human neurons through extracellular signal-regulated kinase activation. Eur J Neurosci 20(12):3222–32. doi:10.1111/j.1460-9568.2004.03800.x
Neeb L, Hellen P, Boehnke C, Hoffmann J, Schuh-Hofer S, Dirnagl U, Reuter U (2011) IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One 6(3):e17360. doi:10.1371/journal.pone.0017360
Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S (2007) Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 129(1-2):155–66. doi:10.1016/j.pain.2006.10.007
Hakim AW, Dong X, Cairns BE (2011) TNFalpha mechanically sensitizes masseter muscle nociceptors by increasing prostaglandin E2 levels. J Neurophysiol 105(1):154–61. doi:10.1152/jn.00730.2010
Hu JH, Yang JP, Liu L, Li CF, Wang LN, Ji FH, Cheng H (2012) Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res 1465:1–9. doi:10.1016/j.brainres.2012.05.020
Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D et al (2004) A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci 24(33):7353–65. doi:10.1523/jneurosci.1850-04.2004
Kiyomoto M, Shinoda M, Okada-Ogawa A, Noma N, Shibuta K, Tsuboi Y, Sessle BJ, Imamura Y et al (2013) Fractalkine signaling in microglia contributes to ectopic orofacial pain following trapezius muscle inflammation. J Neurosci 33(18):7667–80. doi:10.1523/jneurosci.4968-12.2013
Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G et al (2004) Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci 20(9):2294–302. doi:10.1111/j.1460-9568.2004.03709.x
Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ (2007) New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain 129(1-2):64–75. doi:10.1016/j.pain.2006.09.035
Yin Q, Cheng W, Cheng MY, Fan SZ, Shen W (2010) Intrathecal injection of anti-CX3CR1 neutralizing antibody delayed and attenuated pain facilitation in rat tibial bone cancer pain model. Behav Pharmacol 21(7):595–601. doi:10.1097/FBP.0b013e32833e7e2a
Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR (2007) Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun 21(5):642–51. doi:10.1016/j.bbi.2006.11.003
Covasala O, Stirn SL, Albrecht S, De Col R, Messlinger K (2012) Calcitonin gene-related peptide receptors in rat trigeminal ganglion do not control spinal trigeminal activity. J Neurophysiol 108(2):431–40. doi:10.1152/jn.00167.2011
Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S et al (2015) Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. doi:10.1016/j.bbi.2015.10.006
Agalliu I, Xue X, Cushman M, Cornell E, Hsing AW, Kaplan RC, Anastos K, Rajpathak S et al (2013) Detectability and reproducibility of plasma levels of chemokines and soluble receptors. Results Immunol 3:79–84. doi:10.1016/j.rinim.2013.07.001
Suzuki F, Kubota T, Miyazaki Y, Ishikawa K, Ebisawa M, Hirohata S, Ogura T, Mizusawa H et al (2012) Serum level of soluble CX3CL1/fractalkine is elevated in patients with polymyositis and dermatomyositis, which is correlated with disease activity. Arthritis Res Ther 14(2):R48. doi:10.1186/ar3761
Findlay AR, Goyal NA, Mozaffar T (2015) An overview of polymyositis and dermatomyositis. Muscle Nerve 51(5):638–56. doi:10.1002/mus.24566
Acknowledgment
This research was supported by a 2010 Sapere Aude grant from the Danish Council for Independent Research to PG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None to declare
Rights and permissions
About this article
Cite this article
Cairns, B.E., O’Brien, M., Dong, XD. et al. Elevated Fractalkine (CX3CL1) Levels in the Trigeminal Ganglion Mechanically Sensitize Temporalis Muscle Nociceptors. Mol Neurobiol 54, 3695–3706 (2017). https://doi.org/10.1007/s12035-016-9935-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9935-x