Abstract
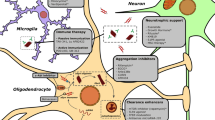
Multiple sclerosis (MS) is a demyelinating neurodegenerative disease, representing a major cause of neurological disability in young adults. Resveratrol is a stilbenoid polyphenol, known to pass blood brain barrier and exhibit antioxidant, anti-inflammatory, and neuroprotective effects in several brain injuries. Cuprizone model of MS is particularly beneficial in studying demyelination/remyelination. Our study examined the potential neuroprotective and pro-remyelination effects of resveratrol in cuprizone-intoxicated C57Bl/6 mice. Mice were fed with chow containing 0.7 % cuprizone for 7 days, followed by 3 weeks on 0.2 % cuprizone diet. Resveratrol (250 mg/kg/day, p.o.) was given for 3 weeks starting from the second week. At the end of the experiment, animals were tested on rotarod to evaluate changes in balance and motor coordination. Mice were then sacrificed to measure the brain content of glutathione, lipid peroxidation products, adenosine triphosphate, and phospho-inhibitory subunit of nuclear factor κB-α. The activities of cytochrome oxidase and superoxide dismutase were also assessed. The gene expression of myelin basic protein, 2′,3′-cyclic nucleotide 3′ phosphodiesterase, oligodendrocyte transcription factor-1 (Olig1), NF-κB p65 subunit, and tumor necrosis factor-α was also estimated. Luxol fast blue/periodic acid-Schiff stained brain sections were blindly scored to assess the myelin status. Resveratrol effectively enhanced motor coordination and balance, reversed cuprizone-induced demyelination, improved mitochondrial function, alleviated oxidative stress, and inhibited NF-κB signaling. Interestingly, resveratrol increased Olig1 expression that is positively correlated to active remyelination. The present study may be the first to indicate a pro-remyelinative effect for resveratrol which might represent a potential additive benefit in treating MS.






Similar content being viewed by others
References
Münzel EJ, Williams A (2013) Promoting remyelination in multiple sclerosis-recent advances. Drugs 73:2017–2029. doi:10.1007/s40265-013-0146-8
Arnett HA, Fancy SPJ, Alberta JA et al (2004) bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306:2111–2115. doi:10.1126/science.1103709
Rodriguez M, Scheithauer BW, Forbes G, Kelly PJ (1993) Oligodendrocyte injury is an early event in lesions of multiple sclerosis. Mayo Clin Proc 68:627–636. doi:10.1016/S0025-6196(12)60597-7
Rodriguez M (2007) Effectors of demyelination and remyelination in the CNS: implications for multiple sclerosis. Brain Pathol 17:219–229. doi:10.1111/j.1750-3639.2007.00065.x
Chang A, Tourtellotte WW, Rudick R, Trapp BD (2002) Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346:165–173. doi:10.1056/NEJMoa010994
Keirstead HS, Blakemore WF (1997) Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol 56:1191–201
Franklin RJM, Ffrench-Constant C (2008) Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855. doi:10.1038/nrn2480
Chang A, Nishiyama A, Peterson J et al (2000) NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20:6404–6412
Meijer DH, Kane MF, Mehta S et al (2012) Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nat Rev Neurosci 13:819–31. doi:10.1038/nrn3386
Zendedel A, Beyer C, Kipp M (2013) Cuprizone-induced demyelination as a tool to study remyelination and axonal protection. J Mol Neurosci 51:567–572. doi:10.1007/s12031-013-0026-4
Ludwin SK (1978) Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Lab Invest 39:597–612
Praet J, Guglielmetti C, Berneman Z et al (2014) Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47:485–505. doi:10.1016/j.neubiorev.2014.10.004
Venturini G (1973) Enzymic activities and sodium, potassium and copper concentrations in mouse brain and liver after cuprizone treatment in vivo. J Neurochem 21:1147–1151. doi:10.1111/j.1471-4159.1973.tb07569.x
Groebe A, Clarner T, Baumgartner W et al (2009) Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum 8:163–174. doi:10.1007/s12311-009-0099-3
Hiremath MMM, Saito Y, Knapp GWW et al (1998) Microglial/macrophage accumulation during cuprizone-induced demyelination in C57Bl/6 mice. J Neuroimmunol 92:38–49. doi:10.1016/S0165-5728(98)00168-4
Rice CM, Sun M, Kemp K et al (2012) Mitochondrial sirtuins—a new therapeutic target for repair and protection in multiple sclerosis. Eur J Neurosci 35:1887–1893. doi:10.1111/j.1460-9568.2012.08150.x
Campbell GR, Ziabreva I, Reeve AK et al (2011) Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol 69:481–492. doi:10.1002/ana.22109
Carlson NG, Rose JW (2006) Antioxidants in multiple sclerosis: do they have a role in therapy? CNS Drugs 20:433–441. doi:10.2165/00023210-200620060-00001
Cuzzola F, Ciurleo R, Giacoppo S et al (2011) Role of resveratrol and its analogues in the treatment of neurodegenerative diseases: focus on recent discoveries. CNS Neurol Disord Drug Targets 10:849–62
Shindler K, Ventura E, Dutt M (2010) Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neurol 30:328–339. doi:10.1097/WNO.0b013e3181f7f833.Oral
Fonseca-Kelly Z, Nassrallah M, Uribe J, et al (2012) Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol 1–9. doi:10.3389/fneur.2012.00084
Bando Y, Ito S, Nagai Y et al (2006) Implications of protease M/neurosin in myelination during experimental demyelination and remyelination. Neurosci Lett 405:175–180. doi:10.1016/j.neulet.2006.06.030
Deacon RMJ (2013) Measuring motor coordination in mice. J Vis Exp e2609. doi:10.3791/2609
Yoshikawa K, Palumbo S, Toscano CD, Bosetti F (2011) Inhibition of 5-lipoxygenase activity in mice during cuprizone-induced demyelination attenuates neuroinflammation, motor dysfunction and axonal damage. Prostaglandins Leukot Essent Fatty Acids 85:43–52. doi:10.1016/j.plefa.2011.04.022
Buege JA, Aust SD (1974) Microsomal lipid peroxidation. In: Methods Enzym. pp 302–310
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522. doi:10.1016/0003-2697(69)90064-5
Navarro A, Boveris A (2004) Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol 287:R1244–R1249. doi:10.1152/ajpregu.00226.2004
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. doi:10.1111/j.1432-1033.1974.tb03714.x
Paradies G, Petrosillo G, Pistolese M, Ruggiero FM (2000) The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Lett 466:323–326. doi:10.1016/S0014-5793(00)01082-6
Lowry OH, Rosebrough NJ, Farr L, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–8. doi:10.1006/meth.2001.1262
Gudi V, Gingele S, Skripuletz T, Stangel M (2014) Glial response during cuprizone-induced de- and remyelination in the CNS: lessons learned. Front Cell Neurosci 8:73. doi:10.3389/fncel.2014.00073
Bando Y, Takakusaki K, Ito S et al (2008) Differential changes in axonal conduction following CNS demyelination in two mouse models. Eur J Neurosci 28:1731–1742. doi:10.1111/j.1460-9568.2008.06474.x
Fulton D, Paez PM, Campagnoni AT (2010) The multiple roles of myelin protein genes during the development of the oligodendrocyte. ASN Neuro 2:e00027. doi:10.1042/AN20090051
Nave K-A (2010) Myelination and the trophic support of long axons. Nat Rev Neurosci 11:275–283. doi:10.1038/nrn2797
Lindner M, Heine S, Haastert K et al (2008) Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol Appl Neurobiol 34:105–114. doi:10.1111/j.1365-2990.2007.00879.x
Baxi EG, DeBruin J, Tosi DM et al (2015) Transfer of myelin-reactive Th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J Neurosci 35:8626–8639. doi:10.1523/JNEUROSCI.3817-14.2015
Roher AE, Weiss N, Kokjohn TA et al (2002) Increased Aβ peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer’ s disease. Biochemistry 41:11080–11090. doi:10.1021/bi026173d
Frid K, Einstein O, Friedman-Levi Y et al (2015) Aggregation of MBP in chronic demyelination. Ann Clin Transl Neurol 2:711–721. doi:10.1002/acn3.207
Gudi V, Moharregh-Khiabani D, Skripuletz T et al (2009) Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res 1283:127–138. doi:10.1016/j.brainres.2009.06.005
Targett MP, Sussman J, Scolding N et al (1996) Failure to achieve remyelination of demyelinated rat axons following transplantation of glial cells obtained from the adult human brain. Neuropathol Appl Neurobiol 22:199–206
Boyd A, Zhang H, Williams A (2013) Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol 125:841–859. doi:10.1007/s00401-013-1112-y
Dai J, Bercury KK, Ahrendsen JT, Macklin WB (2015) Olig1 function is required for oligodendrocyte differentiation in the mouse brain. J Neurosci 35:4386–4402. doi:10.1523/JNEUROSCI.4962-14.2015
El Waly B, Macchi M, Cayre M, Durbec P (2014) Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci 8:1–22. doi:10.3389/fnins.2014.00145
Ziabreva I, Campbell G, Rist J et al (2010) Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes. Glia 58:1827–1837. doi:10.1002/glia.21052
Nguyen NTQ, Ooi L, Piller SC, Münch G (2013) Proenergetic effects of resveratrol in the murine neuronal cell line neuro2a. Mol Nutr Food Res 57:1901–1907. doi:10.1002/mnfr.201300145
Csiszar A, Labinskyy N, Pinto JT et al (2009) Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Hear Circ Physiol 297:H13–H20. doi:10.1152/ajpheart.00368.2009
Price NL, Gomes AP, Ling AJY et al (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. doi:10.1016/j.cmet.2012.04.003
Mc Guire C, Prinz M, Beyaert R, van Loo G (2013) Nuclear factor kappa B (NF-KappaB) in multiple sclerosis pathology. Trends Mol Med 19:604–613. doi:10.1016/j.molmed.2013.08.001
Vakilzadeh G, Khodagholi F, Ghadiri T et al (2015) The effect of melatonin on behavioral, molecular, and histopathological changes in cuprizone model of demyelination. Mol Neurobiol. doi:10.1007/s12035-015-9404-y
Camandola S, Mattson MP (2007) NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets 11:123–132. doi:10.1517/14728222.11.2.123
Raasch J, Zeller N, Van Loo G et al (2011) IκB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-KappaB in the central nervous system. Brain 134:1184–1198. doi:10.1093/brain/awq359
Voß EV, Škuljec J, Gudi V et al (2012) Characterisation of microglia during de- and remyelination: can they create a repair promoting environment? Neurobiol Dis 45:519–528. doi:10.1016/j.nbd.2011.09.008
Singh NP, Hegde VL, Hofseth LJ et al (2007) Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol 72:1508–1521. doi:10.1124/mol.107.038984.compounds
Skripuletz T, Miller E, Moharregh-Khiabani D et al (2010) Beneficial effects of minocycline on cuprizone induced cortical demyelination. Neurochem Res 35:1422–1433. doi:10.1007/s11064-010-0202-7
Hibbits N, Pannu R, Wu TJ, Armstrong RC (2009) Cuprizone demyelination of the corpus callosum in mice correlates with altered social interaction and impaired bilateral sensorimotor coordination. ASN Neuro 1:153–164. doi:10.1042/AN20090032
Bagriyanik HA, Ersoy N, Cetinkaya C et al (2014) The effects of resveratrol on chronic constriction injury of sciatic nerve in rats. Neurosci Lett 561:123–127. doi:10.1016/j.neulet.2013.12.056
Acknowledgments
The authors are grateful to Dr. HebatAllah A. Amin, MD (Department of Pathology, Forensic Medicine Authority, Ministry of Justice, Cairo, Egypt), for her valuable assistance in histopathological examination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Funding
This research was officially supported by the Faculty of Pharmacy, Cairo University.
Rights and permissions
About this article
Cite this article
Ghaiad, H.R., Nooh, M.M., El-Sawalhi, M.M. et al. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol Neurobiol 54, 3219–3229 (2017). https://doi.org/10.1007/s12035-016-9891-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9891-5