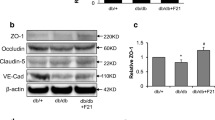
Abstract
Diabetes mellitus (DM) is a metabolic disorder associated with micro- and macrovascular alterations that contribute to the cognitive impairment observed in diabetic patients. Signs of breakdown of the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB) have been found in patients and animal models of DM. Breakdown of the BBB and BCSFB can lead to disruptions in cerebral homeostasis and eventually neural dysfunction and degeneration. However, our understanding of the biochemistry underlying barrier protein modifications is incomplete. Herein, we evaluated changes in the levels of specific proteins in the BBB (occludin, claudin-5, ZO-1, and aquaporin-4) and BCSFB (claudin-2 and aquaporin-1) in the hippocampus of diabetic rats, and we also investigated the functional alterations in these barriers. In addition, we evaluated the ability of exendin-4 (EX-4), a glucagon-like peptide-1 agonist that can cross the BBB to reverse the functional and biochemical modifications observed in these animals. We observed a decrease in BBB proteins (except ZO-1) in diabetic rats, whereas the EX-4 treatment recovered the occludin and aquaporin-4 levels. Similarly, we observed a decrease in BCSFB proteins in diabetic rats, whereas EX-4 reversed such changes. EX-4 also reversed alterations in the permeability of the BBB and BCSFB in diabetic rats. Additionally, altered cognitive parameters in diabetic rats were improved by EX-4. These data further our understanding of the alterations in the central nervous system caused by DM, particularly changes in the proteins and permeability of the brain barriers, as well as cognitive dysfunction. Furthermore, these data suggest a role for EX-4 in therapeutic strategies for cognitive dysfunction in DM.





Similar content being viewed by others
References
Bornstein NM, Brainin M, Guekht A, Skoog I, Korczyn AD (2014) Diabetes and the brain: issues and unmet needs. Neurological Sci: Off J Italian Neurological Society Italian Society Clin Neurophysiology 35(7):995–1001. doi:10.1007/s10072-014-1797-2
Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F et al (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88(2):E14–22
Huber JD, VanGilder RL, Houser KA (2006) Streptozotocin-induced diabetes progressively increases blood–brain barrier permeability in specific brain regions in rats. Am J Physiology Heart Circ Physiology 291(6):H2660–2668. doi:10.1152/ajpheart.00489.2006
Biessels GJ, Reijmer YD (2014) Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 63(7):2244–2252. doi:10.2337/db14-0348
van Duinkerken E, Schoonheim MM, Sanz-Arigita EJ RGIJ, Moll AC, Snoek FJ, Ryan CM, Klein M, Diamant M et al (2012) Resting-state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes 61(7):1814–1821. doi:10.2337/db11-1358
Ramos-Rodriguez JJ, Infante-Garcia C, Galindo-Gonzalez L, Garcia-Molina Y, Lechuga-Sancho A, Garcia-Alloza M (2015) Increased spontaneous central bleeding and cognition impairment in APP/PS1 mice with poorly controlled diabetes mellitus. Mol Neurobiol. doi:10.1007/s12035-015-9311-2
Prasad S, Sajja RK, Naik P, Cucullo L (2014) Diabetes mellitus and blood–brain barrier dysfunction: an overview. J Pharmacovigilance 2(2):125. doi:10.4172/2329-6887.1000125
Mogi M, Horiuchi M (2011) Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ J 75(5):1042–1048
Engelhardt B, Sorokin L (2009) The blood–brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31(4):497–511. doi:10.1007/s00281-009-0177-0
Coisne C, Engelhardt B (2011) Tight junctions in brain barriers during central nervous system inflammation. Antioxidants Redox Signaling 15(5):1285–1303. doi:10.1089/ars.2011.3929
Krueger M, Bechmann I, Immig K, Reichenbach A, Hartig W, Michalski D (2015) Blood–brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cerebral Blood Flow Metabolism: Off J Int Soc Cerebral Blood Flow Metabolism 35(2):292–303. doi:10.1038/jcbfm.2014.199
Marchi N, Granata T, Ghosh C, Janigro D (2012) Blood–brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia 53(11):1877–1886. doi:10.1111/j.1528-1167.2012.03637.x
Daneman R (2012) The blood–brain barrier in health and disease. Ann Neurol 72(5):648–672. doi:10.1002/ana.23648
Benga O, Huber VJ (2012) Brain water channel proteins in health and disease. Mol Asp Med 33(5–6):562–578. doi:10.1016/j.mam.2012.03.008
Badaut J, Fukuda AM, Jullienne A, Petry KG (2014) Aquaporin and brain diseases. Biochim Biophys Acta 1840(5):1554–1565. doi:10.1016/j.bbagen.2013.10.032
Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
Unger J (2013) Rationale use of GLP-1 receptor agonists in patients with type 1 diabetes. Current Diabetes Reports 13(5):663–668. doi:10.1007/s11892-013-0404-x
Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ (2005) beta-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 54(2):482–491
Pettus J, Hirsch I, Edelman S (2013) GLP-1 agonists in type 1 diabetes. Clin Immunol 149(3):317–323. doi:10.1016/j.clim.2013.04.006
Holscher C (2014) Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinology 221(1):T31–41. doi:10.1530/JOE-13-0221
Huang HJ, Chen YH, Liang KC, Jheng YS, Jhao JJ, Su MT, Lee-Chen GJ, Hsieh-Li HM (2012) Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS One 7(7), e39656. doi:10.1371/journal.pone.0039656
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60
Kozler P, Pokorny J (2003) Evans blue distribution in the rate brain after intracarotid injection with the blood–brain barrier intact and open to osmosis. Sb Lek 104(3):255–262
Liu X, Wang Z, Wang P, Yu B, Liu Y, Xue Y (2013) Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complementary Alternative Med 13:187. doi:10.1186/1472-6882-13-187
Netto CB, Conte S, Leite MC, Pires C, Martins TL, Vidal P, Benfato MS, Giugliani R et al (2006) Serum S100B protein is increased in fasting rats. Arch Med Res 37(5):683–686. doi:10.1016/j.arcmed.2005.11.005
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biological Chem 193(1):265–275
Durgawale P, Kanase S, Shukla PS, Sontakke S (2005) A sensitive and economical modified method for estimation of cerebrospinal fluid proteins. Indian J Clin Biochem: IJCB 20(2):174–177. doi:10.1007/BF02867422
Zanotto C, Abib RT, Batassini C, Tortorelli LS, Biasibetti R, Rodrigues L, Nardin P, Hansen F et al (2013) Non-specific inhibitors of aquaporin-4 stimulate S100B secretion in acute hippocampal slices of rats. Brain Res 1491:14–22. doi:10.1016/j.brainres.2012.10.065
Ahmad W (2013) Overlapped metabolic and therapeutic links between Alzheimer and diabetes. Mol Neurobiol 47(1):399–424. doi:10.1007/s12035-012-8352-z
Taguchi A (2009) Vascular factors in diabetes and Alzheimer’s disease. J Alzheimer’s Disease: JAD 16(4):859–864. doi:10.3233/JAD-2009-0975
Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, Masliah E (2008) Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res 86(15):3265–3274. doi:10.1002/jnr.21787
Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M et al (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A 107(15):7036–7041. doi:10.1073/pnas.1000645107
Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH (2002) Ageing and diabetes: implications for brain function. Eur J Pharmacol 441(1–2):1–14
de Senna PN, Ilha J, Baptista PP, do Nascimento PS, Leite MC, Paim MF, Goncalves CA, Achaval M et al (2011) Effects of physical exercise on spatial memory and astroglial alterations in the hippocampus of diabetic rats. Metab Brain Dis 26(4):269–279. doi:10.1007/s11011-011-9262-x
Serlin Y, Levy J, Shalev H (2011) Vascular pathology and blood–brain barrier disruption in cognitive and psychiatric complications of type 2 diabetes mellitus. Cardiovascular Psych Neurol 2011:609202. doi:10.1155/2011/609202
Aggarwal A, Khera A, Singh I, Sandhir R (2014) S-nitrosoglutathione prevents blood–brain barrier disruption associated with increased matrix metalloproteinase-9 activity in experimental diabetes. J Neurochem. doi:10.1111/jnc.12939
VanGilder RL, Kelly KA, Chua MD, Ptachcinski RL, Huber JD (2009) Administration of sesamol improved blood–brain barrier function in streptozotocin-induced diabetic rats. Exp Brain Res 197(1):23–34. doi:10.1007/s00221-009-1866-6
Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD (2007) Increased blood–brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 50(1):202–211. doi:10.1007/s00125-006-0485-z
Bradbury MW, Lightman SL, Yuen L, Pinter GG (1991) Permeability of blood–brain and blood-nerve barriers in experimental diabetes mellitus in the anaesthetized rat. Exp Physiol 76(6):887–898
Dai J, Vrensen GF, Schlingemann RO (2002) Blood–brain barrier integrity is unaltered in human brain cortex with diabetes mellitus. Brain Res 954(2):311–316
Mooradian AD, Haas MJ, Batejko O, Hovsepyan M, Feman SS (2005) Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes 54(10):2977–2982
Chehade JM, Haas MJ, Mooradian AD (2002) Diabetes-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) expression. Neurochem Res 27(3):249–252
Shao B, Bayraktutan U (2013) Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-beta. Diabetes, Obesity Metabolism 15(11):993–999. doi:10.1111/dom.12120
Sun YN, Liu LB, Xue YX, Wang P (2015) Effects of insulin combined with idebenone on blood–brain barrier permeability in diabetic rats. J Neurosci Res 93(4):666–677. doi:10.1002/jnr.23511
Shimizu F, Sano Y, Tominaga O, Maeda T, Abe MA, Kanda T (2013) Advanced glycation end-products disrupt the blood–brain barrier by stimulating the release of transforming growth factor-beta by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol Aging 34(7):1902–1912. doi:10.1016/j.neurobiolaging.2013.01.012
Arlt S, Kontush A, Zerr I, Buhmann C, Jacobi C, Schroter A, Poser S, Beisiegel U (2002) Increased lipid peroxidation in cerebrospinal fluid and plasma from patients with Creutzfeldt-Jakob disease. Neurobiol Dis 10(2):150–156
Egleton RD, Campos CC, Huber JD, Brown RC, Davis TP (2003) Differential effects of diabetes on rat choroid plexus ion transporter expression. Diabetes 52(6):1496–1501
de Senna PN, Xavier LL, Bagatini PB, Saur L, Galland F, Zanotto C, Bernardi C, Nardin P et al (2015) Physical training improves non-spatial memory, locomotor skills and the blood brain barrier in diabetic rats. Brain Res 1618:75–82. doi:10.1016/j.brainres.2015.05.026
Koves IH, Russo VC, Higgins S, Mishra A, Pitt J, Cameron FJ, Werther GA (2012) An in vitro paradigm for diabetic cerebral oedema and its therapy: a critical role for taurine and water channels. Neurochem Res 37(1):182–192. doi:10.1007/s11064-011-0598-8
Deng J, Zhao F, Yu X, Zhao Y, Li D, Shi H, Sun Y (2014) Expression of aquaporin 4 and breakdown of the blood–brain barrier after hypoglycemia-induced brain edema in rats. PLoS One 9(9), e107022. doi:10.1371/journal.pone.0107022
Nejsum LN, Kwon TH, Marples D, Flyvbjerg A, Knepper MA, Frokiaer J, Nielsen S (2001) Compensatory increase in AQP2, p-AQP2, and AQP3 expression in rats with diabetes mellitus. Am J Physiology Renal Physiology 280(4):F715–726
Nesic O, Lee J, Unabia GC, Johnson K, Ye Z, Vergara L, Hulsebosch CE, Perez-Polo JR (2008) Aquaporin 1—a novel player in spinal cord injury. J Neurochem 105(3):628–640. doi:10.1111/j.1471-4159.2007.05177.x
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4(147), 147ra111. doi:10.1126/scitranslmed.3003748
Nagelhus EA, Ottersen OP (2013) Physiological roles of aquaporin-4 in brain. Physiol Rev 93(4):1543–1562. doi:10.1152/physrev.00011.2013
Ninomiya T (2014) Diabetes mellitus and dementia. Current Diabetes Reports 14(5):487. doi:10.1007/s11892-014-0487-z
Kaya M, Kalayci R, Kucuk M, Arican N, Elmas I, Kudat H, Korkut F (2003) Effect of losartan on the blood–brain barrier permeability in diabetic hypertensive rats. Life Sci 73(25):3235–3244
Jing YH, Chen KH, Kuo PC, Pao CC, Chen JK (2013) Neurodegeneration in streptozotocin-induced diabetic rats is attenuated by treatment with resveratrol. Neuroendocrinology 98(2):116–127. doi:10.1159/000350435
Yamagishi S, Nakamura K, Inoue H, Kikuchi S, Takeuchi M (2005) Serum or cerebrospinal fluid levels of glyceraldehyde-derived advanced glycation end products (AGEs) may be a promising biomarker for early detection of Alzheimer’s disease. Med Hypotheses 64(6):1205–1207. doi:10.1016/j.mehy.2005.01.016
Gunzel D, Yu AS (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93(2):525–569. doi:10.1152/physrev.00019.2012
Harhaj NS, Antonetti DA (2004) Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 36(7):1206–1237. doi:10.1016/j.biocel.2003.08.007
Seufert J, Gallwitz B (2014) The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes, Obesity Metabolism 16(8):673–688. doi:10.1111/dom.12251
Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH (2010) Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem 113(6):1621–1631. doi:10.1111/j.1471-4159.2010.06731.x
Muriach M, Flores-Bellver M, Romero FJ, Barcia JM (2014) Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxidative Med Cell Longev 2014:102158. doi:10.1155/2014/102158
Acknowledgments
This study was supported by the National Council for Scientific and Technological Development (CNPq, Brazil), Ministry of Education (MEC/CAPES, Brazi), State Foundation for Scientific Research of Rio Grande do Sul (FAPERGS), and National Institute of Science and Technology for Excitotoxicity and Neuroprotection (MCT/INCTEN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications no. 80–23), and all procedures were previously approved by the local Animal Care Ethical Committee (CEUA-UFRGS; project number 24076). All efforts were made to minimize animal suffering and reduce the number of animals used.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zanotto, C., Simão, F., Gasparin, M.S. et al. Exendin-4 Reverses Biochemical and Functional Alterations in the Blood–Brain and Blood–CSF Barriers in Diabetic Rats. Mol Neurobiol 54, 2154–2166 (2017). https://doi.org/10.1007/s12035-016-9798-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9798-1