Abstract
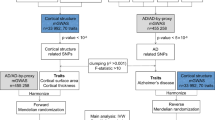
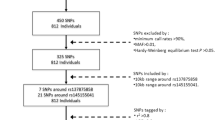
The cluster of differentiation 33 (CD33) has been proved as a susceptibility locus associated with late-onset Alzheimer’s disease (LOAD) based on recent genetic studies. Numerous studies have shown that multiple neuroimaging measures are potent predictors of AD risk and progression, and these measures are also affected by genetic variations in AD. Figuring out the association between CD33 genetic variations and AD-related brain atrophy may shed light on the underlying mechanisms of CD33-related AD pathogenesis. Thus, we investigated the influence of CD33 genotypes on AD-related brain atrophy to clarify the possible means by which CD33 impacts AD. A total of 48 individuals with probable AD, 483 mild cognitive impairment, and 281 cognitively normal controls were recruited from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset. We investigated the influence of CD33 SNPs on hippocampal volume, parahippocampal gyrus volume, posterior cingulate volume, middle temporal volume, hippocampus CA1 subregion volume, and entorhinal cortex thickness. We found that brain regions significantly affected by CD33 genetic variations were restricted to hippocampal and parahippocampal gyrus in hybrid population, which were further validated in subpopulation (MCI and NC) analysis. These findings reaffirm the importance of the hippocampal and parahippocampal gyrus in AD pathogenesis, and present evidences for the CD33 variations influence on the atrophy of specific AD-related brain structures. Our findings raise the possibility that CD33 polymorphisms contribute to the AD risk by altering the neuronal degeneration of hippocampal and parahippocampal gyrus.


Similar content being viewed by others
References
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. Lancet 377:1019–1031
Chan KY, Wang W, Wu JJ et al (2013) Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 381:2016–2023
Jiang T, Yu JT, Hu N, Tan MS, Zhu XC, Tan L (2014) CD33 in Alzheimer’s disease. Mol Neurobiol 49:529–535
Hollingworth P, Harold D, Sims R et al (2011) Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43:429–435
Bertram L, Lange C, Mullin K et al (2008) Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet 83:623–632
Naj AC, Jun G, Beecham GW et al (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 43:436–441
Carrasquillo MM, Belbin O, Hunter TA et al (2011) Replication of EPHA1 and CD33 associations with late-onset Alzheimer’s disease: a multi-centre case-control study. Mol Neurodegener 6:54
Chung SJ, Lee JH, Kim SY et al (2013) Association of GWAS top hits with late-onset Alzheimer disease in Korean population. Alzheimer Dis Assoc Disord 27:250–257
Tan L, Yu JT, Zhang W et al (2013) Association of GWAS-linked loci with late-onset Alzheimer’s disease in a northern Han Chinese population. Alzheimers Dement 9:546–553
Griciuc A, Serrano-Pozo A, Parrado AR et al (2013) Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78:631–643
Bradshaw EM, Chibnik LB, Keenan BT et al (2013) CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci 16:848–850
Jack CR Jr, Knopman DS, Jagust WJ et al (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128
Liu Y, Yu JT, Wang HF et al (2015) APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 86:127–134
Liu Y, Tan L, Wang HF, et al. (2015) Multiple Effect of APOE Genotype on Clinical and Neuroimaging Biomarkers Across Alzheimer’s Disease Spectrum. Mol Neurobiol.
Chauhan G, Adams HH, Bis JC et al (2015) Association of Alzheimer’s disease GWAS loci with MRI markers of brain aging. Neurobiol Aging 36:1765, e1767-1716
Saykin AJ, Shen L, Foroud TM et al (2010) Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimers Dement 6:265–273
Fischl B, Salat DH, van der Kouwe AJ et al (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23(Suppl 1):S69–84
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055
Liu Y, Yu JT, Wang HF et al (2014) Association between NME8 locus polymorphism and cognitive decline, cerebrospinal fluid and neuroimaging biomarkers in Alzheimer’s disease. PLoS One 9:e114777
Biffi A, Anderson CD, Desikan RS et al (2010) Genetic variation and neuroimaging measures in Alzheimer disease. Arch Neurol 67:677–685
Wang WY, Yu JT, Liu Y et al (2015) Voxel-based meta-analysis of grey matter changes in Alzheimer’s disease. Transl Neurodegener 4:6
Chang YT, Huang CW, Chang YH et al (2015) Amyloid burden in the hippocampus and default mode network: relationships with gray matter volume and cognitive performance in mild stage Alzheimer disease. Medicine (Baltimore) 94:e763
Hu N, Tan MS, Sun L et al (2014) Decreased expression of CD33 in peripheral mononuclear cells of Alzheimer’s disease patients. Neurosci Lett 563:51–54
Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K (2006) Role of toll-like receptor signalling in Abeta uptake and clearance. Brain 129:3006–3019
Sheng JG, Mrak RE, Griffin WS (1995) Microglial interleukin-1 alpha expression in brain regions in Alzheimer’s disease: correlation with neuritic plaque distribution. Neuropathol Appl Neurobiol 21:290–301
Zeineh MM, Chen Y, Kitzler HH, Hammond R, Vogel H, Rutt BK (2015) Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol Aging 36:2483–2500
Acknowledgments
Data collection and sharing was funded by the ADNI (National Institutes of Health U01 AG024904). ADNI is funded by the National Institute on Aging; the National Institute of Biomedical Imaging and Bioengineering; the Alzheimer’s Association; the Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Co., F. Hoffmann-LaRoche Ltd and Genetech, Inc.; GE Healthcare; Innogenetics, NV; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development LLC; Medpace, Inc; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Novartis Pharmaceuticals, Co., Pfizer, Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Co. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private-sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization was the Northern California Institute for Research and Education, and the study was coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This work was also supported by grants from the National Natural Science Foundation of China (81471309, 81171209, 81371406, 81501103, 81571245), the Shandong Provincial Outstanding Medical Academic Professional Program, Qingdao Key Health Discipline Development Fund, Qingdao Outstanding Health Professional Development Fund, and Shandong Provincial Collaborative Innovation Center for Neurodegenerative Disorders.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Wen-Ying Wang and Ying Liu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 52 kb)
Rights and permissions
About this article
Cite this article
Wang, WY., Liu, Y., Wang, HF. et al. Impacts of CD33 Genetic Variations on the Atrophy Rates of Hippocampus and Parahippocampal Gyrus in Normal Aging and Mild Cognitive Impairment. Mol Neurobiol 54, 1111–1118 (2017). https://doi.org/10.1007/s12035-016-9718-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9718-4