Abstract
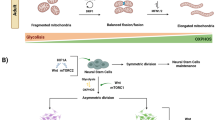
Pluripotent stem cells (PSCs) are powerful cellular tools that can generate all the different cell types of the body, and thus overcome the often limited access to human disease tissues; this becomes highly relevant when aiming to investigate cellular (dys)function in diseases affecting the central nervous system. Recent studies have demonstrated that PSC and differentiated cells show altered mitochondrial function and metabolic profiles and production of reactive oxygen species. This raises an emerging paradigm about the role of mitochondria in stem cell biology and urges the need to identify mitochondrial pathways involved in these processes. In this respect, this review focuses on the metabolic profile of PSC and how mitochondrial function can influence the reprogramming and differentiation processes. Indeed, both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) favor the glycolytic pathway as a major source of energy production over oxidative phosphorylation. PSC mitochondria are characterized by a spherical shape, low copy number of mitochondrial DNA, and a hyperpolarized state. Indeed, mitochondria appear to have a crucial role in reprogramming iPSC, in the maintenance of a pluripotent state, and in differentiation. Moreover, an increase in mitochondrial oxidative phosphorylation has to occur for differentiation to succeed. Therefore, in vitro differentiation of neural stem cells (NSCs) into neurons can be compromised if those mechanisms are impaired. Future research should shed light on how mitochondrial impairment occurring in pre differentiation neural stages (e.g., in NSC or premature neurons) may contribute for the etiopathogenesis of neurodevelopmental and neurological disorders.

Similar content being viewed by others
Abbreviations
- NSC:
-
Neural stem cells
- PSC:
-
Pluripotent stem cells
- iPSC:
-
Induced pluripotent stem cells
- hESC:
-
Human embryonic stem cells
- EBs:
-
Embryoid bodies
- SSEA:
-
Stage Specific Embryonic Antigens
- Sox2:
-
High-mobility-group (HMG)-box protein-2
- Klf4:
-
Kruppel-like factor-4
- cDNAs:
-
Complementary DNAs
- ROS:
-
Reactive oxygen species
- PPP:
-
Pentose phosphate pathway
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate reduced
- GSH:
-
Reduced glutathione
- acetyl-CoA:
-
Acetyl-coenzyme A
- PDH:
-
Pyruvate dehydrogenase
- OXPHOS:
-
Oxidative phosphorylation
- mtDNA:
-
Mitochondrial DNA
- ΔΨm :
-
Mitochondrial membrane potential
- MEFs:
-
Mouse embryonic fibroblasts
- LDH:
-
Lactate dehydrogenase
- HIF1α:
-
Hypoxia-inducible factor one alpha
- PDK3:
-
PDH kinase 3
- PKM2:
-
Pyruvate kinase M2 isoform
- TCA:
-
Tricarboxylic acid
- GSK3:
-
Glycogen synthase kinase-3
- MEK/MAPK:
-
Mitogen-activated protein kinase
- OCR:
-
Oxygen consumption rate
- FCCP:
-
Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- mESC:
-
Mouse ESC
- COX:
-
Cyclooxygenase
- LOX:
-
Lipoxygenase
- PTK:
-
Palmityl trifluoromethyl ketone
- SAM:
-
S-adenosyl methionine
- Thr:
-
Threonine
- Tdh:
-
Threonine dehydrogenase
- H3K4me3:
-
Histone H3 lysine 4
- UCP:
-
Uncoupling proteins
- SOD:
-
Superoxide dismutase
- GPX:
-
Glutathione peroxidase
- Nox:
-
NADPH oxidase
- Drp1:
-
Dynamin-related protein
- Fis1:
-
Mitochondrial fission 1
- Mfn:
-
Mitofusin
- Opa1:
-
Optic atrophy type 1
- Gfer:
-
Growth factor erv1-like
- TFAM:
-
Mitochondrial transcription factor A
- PGC-1α:
-
Peroxisome proliferator-activated receptor-c coactivator-1α
- POLG:
-
DNA polymerase γ
- NRF-1:
-
Nuclear respiratory factor-1
- mTOR:
-
Mammalian target of rapamycin
- YY1:
-
Transcription factor yang 1
- AD:
-
Alzheimer Disease
- PD:
-
Parkinson Disease
- HD:
-
Huntington Disease
- NPCs:
-
Neural progenitor cells
- FRDA:
-
Friedreich ataxia
- FXN :
-
Frataxin gene
- LRRK2:
-
Leucine-rich repeat kinase-2
- PINK:
-
PTEN-induced putative kinase
- DARPP-32:
-
Dopamine- and cAMP-regulated neuronal phosphoprotein
- BDNF:
-
Brain-derived neurotrophic factor
- 3-MA:
-
3-methyladenine
- GST:
-
Glutathione S-transferase
- Prx:
-
Peroxiredoxin
- FAD:
-
Familial form of AD
- PS:
-
Presenilin
- APP:
-
Amyloid precursor protein.
References
Dyall SD, Brown MT, Johnson PJ (2004) Ancient invasions: from endosymbionts to organelles. Science 304(5668):253–257. doi:10.1126/science.1094884
Ribeiro M, Rosenstock TR, Oliveira AM, Oliveira CR, Rego AC (2014) Insulin and IGF-1 improve mitochondrial function in a PI-3K/Akt-dependent manner and reduce mitochondrial generation of reactive oxygen species in Huntington’s disease knock-in striatal cells. Free Radic Biol Med 74:129–144. doi:10.1016/j.freeradbiomed.2014.06.023
Varum S, Momcilovic O, Castro C, Ben-Yehudah A, Ramalho-Santos J, Navara CS (2009) Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res 3(2–3):142–156. doi:10.1016/j.scr.2009.07.002
Liu Z, Wen X, Wang H, Zhou J, Zhao M, Lin Q, Wang Y, Li J et al (2013) Molecular imaging of induced pluripotent stem cell immunogenicity with in vivo development in ischemic myocardium. PLoS One 8(6):e66369. doi:10.1371/journal.pone.0066369
Son MJ, Jeong BR, Kwon Y, Cho YS (2013) Interference with the mitochondrial bioenergetics fuels reprogramming to pluripotency via facilitation of the glycolytic transition. Int J Biochem Cell Biol 45(11):2512–2518. doi:10.1016/j.biocel.2013.07.023
Chen CT, Hsu SH, Wei YH (2010) Upregulation of mitochondrial function and antioxidant defense in the differentiation of stem cells. Biochim Biophys Acta 1800(3):257–263. doi:10.1016/j.bbagen.2009.09.001
Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J (2010) The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28(4):721–733. doi:10.1002/stem.404
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292(5819):154–156
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78(12):7634–7638
Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 6(2):88–95
Stewart MH, Bosse M, Chadwick K, Menendez P, Bendall SC, Bhatia M (2006) Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat Methods 3(10):807–815. doi:10.1038/nmeth939
Kannagi R, Cochran NA, Ishigami F, Hakomori S, Andrews PW, Knowles BB, Solter D (1983) Stage-specific embryonic antigens (SSEA-3 and −4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J 2(12):2355–2361
Andrews PW, Casper J, Damjanov I, Duggan-Keen M, Giwercman A, Hata J, von Keitz A, Looijenga LH et al (1996) Comparative analysis of cell surface antigens expressed by cell lines derived from human germ cell tumours. Int J Cancer 66(6):806–816. doi:10.1002/(SICI)1097-0215(19960611)66:6<806::AID-IJC17>3.0.CO;2-0
Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O (2007) Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells 25(9):2257–2268. doi:10.1634/stemcells.2006-0744
Andrews PW, Banting G, Damjanov I, Arnaud D, Avner P (1984) Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma 3(4):347–361
Zhao W, Ji X, Zhang F, Li L, Ma L (2012) Embryonic stem cell markers. Molecules 17(6):6196–6236. doi:10.3390/molecules17066196
Stojkovic M, Lako M, Strachan T, Murdoch A (2004) Derivation, growth and applications of human embryonic stem cells. Reproduction 128(3):259–267. doi:10.1530/rep.1.00243
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. doi:10.1016/j.cell.2007.11.019
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. doi:10.1016/j.cell.2006.07.024
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448(7151):313–317. doi:10.1038/nature05934
Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Guo CL et al (2009) iPS cells produce viable mice through tetraploid complementation. Nature 461(7260):86–90. doi:10.1038/nature08267
Kang L, Wang J, Zhang Y, Kou Z, Gao S (2009) iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 5(2):135–138. doi:10.1016/j.stem.2009.07.001
Okita K, Hong H, Takahashi K, Yamanaka S (2010) Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nat Protoc 5(3):418–428. doi:10.1038/nprot.2009.231
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322(5903):949–953. doi:10.1126/science.1164270
Sommer AG, Rozelle SS, Sullivan S, Mills JA, Park SM, Smith BW, Iyer AM, French DL, Kotton DN, Gadue P, Murphy GJ, Mostoslavsky G (2012) Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J Vis Exp (68). doi:10.3791/4327
Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G (2009) Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27(3):543–549. doi:10.1634/stemcells.2008-1075
Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M (2009) Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 85(8):348–362
Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W et al (2009) piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458(7239):766–770. doi:10.1038/nature07863
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324(5928):797–801. doi:10.1126/science.1172482
Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G et al (2009) Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4(5):381–384. doi:10.1016/j.stem.2009.04.005
Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W et al (2011) Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 8(4):376–388. doi:10.1016/j.stem.2011.03.001
Pan G, Thomson JA (2007) Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res 17(1):42–49. doi:10.1038/sj.cr.7310125
Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O et al (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5(1):111–123. doi:10.1016/j.stem.2009.06.008
Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR (2009) Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One 4(9):e7076. doi:10.1371/journal.pone.0007076
Prigione A, Adjaye J (2010) Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol 54(11–12):1729–1741. doi:10.1387/ijdb.103198ap
Razak SR, Ueno K, Takayama N, Nariai N, Nagasaki M, Saito R, Koso H, Lai CY et al (2013) Profiling of microRNA in human and mouse ES and iPS cells reveals overlapping but distinct microRNA expression patterns. PLoS One 8(9):e73532. doi:10.1371/journal.pone.0073532
Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K et al (2011) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 30(24):4860–4873. doi:10.1038/emboj.2011.401
Panopoulos AD, Yanes O, Ruiz S, Kida YS, Diep D, Tautenhahn R, Herrerias A, Batchelder EM et al (2012) The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res 22(1):168–177. doi:10.1038/cr.2011.177
Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CA, Ramalho-Santos J, Van Houten B, Schatten G (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6(6):e20914. doi:10.1371/journal.pone.0020914
Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT et al (2014) Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 14(5):592–605. doi:10.1016/j.stem.2014.02.012
Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, Blumlein K, Wanker EE et al (2014) HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells 32(2):364–376. doi:10.1002/stem.1552
Mathieu J, Zhang Z, Nelson A, Lamba DA, Reh TA, Ware C, Ruohola-Baker H (2013) Hypoxia induces re-entry of committed cells into pluripotency. Stem Cells 31(9):1737–1748. doi:10.1002/stem.1446
Roche TE, Baker JC, Yan X, Hiromasa Y, Gong X, Peng T, Dong J, Turkan A et al (2001) Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog Nucleic Acid Res Mol Biol 70:33–75
Pecqueur C, Alves-Guerra MC, Gelly C, Levi-Meyrueis C, Couplan E, Collins S, Ricquier D, Bouillaud F et al (2001) Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem 276(12):8705–8712. doi:10.1074/jbc.M006938200
Pecqueur C, Alves-Guerra C, Ricquier D, Bouillaud F (2009) UCP2, a metabolic sensor coupling glucose oxidation to mitochondrial metabolism? IUBMB Life 61(7):762–767. doi:10.1002/iub.188
Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C et al (2010) Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol 6(6):411–417. doi:10.1038/nchembio.364
Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T et al (2013) Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339(6116):222–226. doi:10.1126/science.1226603
Wang J, Alexander P, Wu L, Hammer R, Cleaver O, McKnight SL (2009) Dependence of mouse embryonic stem cells on threonine catabolism. Science 325(5939):435–439. doi:10.1126/science.1173288
Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G, Aburatani H, Kume K et al (2014) Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab 19(5):780–794. doi:10.1016/j.cmet.2014.03.017
Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC (2007) Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120(Pt 22):4025–4034. doi:10.1242/jcs.016972
St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP (2005) The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells 7(3):141–153. doi:10.1089/clo.2005.7.141
Birket MJ, Orr AL, Gerencser AA, Madden DT, Vitelli C, Swistowski A, Brand MD, Zeng X (2011) A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J Cell Sci 124(Pt 3):348–358. doi:10.1242/jcs.072272
Suhr ST, Chang EA, Tjong J, Alcasid N, Perkins GA, Goissis MD, Ellisman MH, Perez GI et al (2010) Mitochondrial rejuvenation after induced pluripotency. PLoS One 5(11):e14095. doi:10.1371/journal.pone.0014095
Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C et al (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14(2):264–271. doi:10.1016/j.cmet.2011.06.011
Zeuschner D, Mildner K, Zaehres H, Scholer HR (2010) Induced pluripotent stem cells at nanoscale. Stem Cells Dev 19(5):615–620. doi:10.1089/scd.2009.0159
Kelly RD, Sumer H, McKenzie M, Facucho-Oliveira J, Trounce IA, Verma PJ, St John JC (2013) The effects of nuclear reprogramming on mitochondrial DNA replication. Stem Cell Rev 9(1):1–15. doi:10.1007/s12015-011-9318-7
Wanet A, Arnould T, Najimi M, Renard P (2015) Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev 24(17):1957–1971. doi:10.1089/scd.2015.0117
Wang L, Ye X, Zhao Q, Zhou Z, Dan J, Zhu Y, Chen Q, Liu L (2014) Drp1 is dispensable for mitochondria biogenesis in induction to pluripotency but required for differentiation of embryonic stem cells. Stem Cells Dev 23(20):2422–2434. doi:10.1089/scd.2014.0059
Vazquez-Martin A, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Vellon L, Menendez JA (2012) Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging (Albany NY) 4(6):393–401
Todd LR, Damin MN, Gomathinayagam R, Horn SR, Means AR, Sankar U (2010) Growth factor erv1-like modulates Drp1 to preserve mitochondrial dynamics and function in mouse embryonic stem cells. Mol Biol Cell 21(7):1225–1236. doi:10.1091/mbc.E09-11-0937
Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A (2007) Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med 4(Suppl 1):S60–67. doi:10.1038/ncpcardio0766
Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH (2008) Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26(4):960–968. doi:10.1634/stemcells.2007-0509
Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, do Park J, Park KS, Lee HK (2006) Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun 348(4):1472–1478. doi:10.1016/j.bbrc.2006.08.020
Holmuhamedov E, Jahangir A, Bienengraeber M, Lewis LD, Terzic A (2003) Deletion of mtDNA disrupts mitochondrial function and structure, but not biogenesis. Mitochondrion 3(1):13–19. doi:10.1016/S1567-7249(03)00053-9
Lloyd RE, Lee JH, Alberio R, Bowles EJ, Ramalho-Santos J, Campbell KH, St John JC (2006) Aberrant nucleo-cytoplasmic cross-talk results in donor cell mtDNA persistence in cloned embryos. Genetics 172(4):2515–2527. doi:10.1534/genetics.105.055145
Spitkovsky D, Sasse P, Kolossov E, Bottinger C, Fleischmann BK, Hescheler J, Wiesner RJ (2004) Activity of complex III of the mitochondrial electron transport chain is essential for early heart muscle cell differentiation. FASEB J 18(11):1300–1302. doi:10.1096/fj.03-0520fje
Prigione A, Hossini AM, Lichtner B, Serin A, Fauler B, Megges M, Lurz R, Lehrach H et al (2011) Mitochondrial-associated cell death mechanisms are reset to an embryonic-like state in aged donor-derived iPS cells harboring chromosomal aberrations. PLoS One 6(11):e27352. doi:10.1371/journal.pone.0027352
Schieke SM, Ma M, Cao L, McCoy JP Jr, Liu C, Hensel NF, Barrett AJ, Boehm M et al (2008) Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem 283(42):28506–28512. doi:10.1074/jbc.M802763200
Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P (2007) mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450(7170):736–740. doi:10.1038/nature06322
Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M (2010) Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28(4):661–673. doi:10.1002/stem.307
Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S et al (2008) Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells 26(2):455–464. doi:10.1634/stemcells.2007-0628
Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U (2011) Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 29(3):486–495. doi:10.1002/stem.590
Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, Kim JW, Yustein JT et al (2005) Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol 25(14):6225–6234. doi:10.1128/MCB.25.14.6225-6234.2005
Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F et al (2014) Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158(6):1254–1269. doi:10.1016/j.cell.2014.08.029
Crespo FL, Sobrado VR, Gomez L, Cervera AM, McCreath KJ (2010) Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells 28(7):1132–1142. doi:10.1002/stem.441
Block K, Gorin Y, Abboud HE (2009) Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106(34):14385–14390. doi:10.1073/pnas.0906805106
Heinrichs RW, Zakzanis KK (1998) Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12(3):426–445
Andreasen NC, Paradiso S, O’Leary DS (1998) “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 24(2):203–218
Rubinov M, Bullmore E (2013) Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci 15(3):339–349
Yao JK, Leonard S, Reddy RD (2004) Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull 30(4):923–934
Robicsek O, Karry R, Petit I, Salman-Kesner N, Muller FJ, Klein E, Aberdam D, Ben-Shachar D (2013) Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry 18(10):1067–1076. doi:10.1038/mp.2013.67
Rosenfeld M, Brenner-Lavie H, Ari SG, Kavushansky A, Ben-Shachar D (2011) Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry 69(10):980–988. doi:10.1016/j.biopsych.2011.01.010
Paulsen Bda S, de Moraes MR, Galina A, Souza da Silveira M, dos Santos SC, Drummond H, Nascimento Pozzatto E, Silva H Jr et al (2012) Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant 21(7):1547–1559. doi:10.3727/096368911X600957
Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran I, Abdelrahim M, Matikainen-Ankney B, Chao SH, Mrksich M, Rakic P, Fang G, Zhang B, Yates JR, 3rd, Gage FH (2014) Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. doi:10.1038/mp.2014.22
Gomes CM, Santos R (2013) Neurodegeneration in Friedreich’s ataxia: from defective frataxin to oxidative stress. Oxid Med Cell Longev 2013:487534. doi:10.1155/2013/487534
Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F et al (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271(5254):1423–1427
Liu J, Verma PJ, Evans-Galea MV, Delatycki MB, Michalska A, Leung J, Crombie D, Sarsero JP et al (2011) Generation of induced pluripotent stem cell lines from Friedreich ataxia patients. Stem Cell Rev 7(3):703–713. doi:10.1007/s12015-010-9210-x
Ku S, Soragni E, Campau E, Thomas EA, Altun G, Laurent LC, Loring JF, Napierala M et al (2010) Friedreich’s ataxia induced pluripotent stem cells model intergenerational GAATTC triplet repeat instability. Cell Stem Cell 7(5):631–637. doi:10.1016/j.stem.2010.09.014
Hick A, Wattenhofer-Donze M, Chintawar S, Tropel P, Simard JP, Vaucamps N, Gall D, Lambot L et al (2013) Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich’s ataxia. Dis Model Mech 6(3):608–621. doi:10.1242/dmm.010900
Eigentler A, Boesch S, Schneider R, Dechant G, Nat R (2013) Induced pluripotent stem cells from Friedreich ataxia patients fail to upregulate frataxin during in vitro differentiation to peripheral sensory neurons. Stem Cells Dev 22(24):3271–3282. doi:10.1089/scd.2013.0126
Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M et al (2011) Parkinson’s disease induced pluripotent stem cells with triplication of the alpha-synuclein locus. Nat Commun 2:440. doi:10.1038/ncomms1453
Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B et al (2011) LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8(3):267–280. doi:10.1016/j.stem.2011.01.013
Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D (2011) Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci 31(16):5970–5976. doi:10.1523/JNEUROSCI.4441-10.2011
Soldner F, Laganiere J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI et al (2011) Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146(2):318–331. doi:10.1016/j.cell.2011.06.019
Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K et al (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 3:668. doi:10.1038/ncomms1669
Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, Jimenez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M et al (2012) Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med 4(5):380–395. doi:10.1002/emmm.201200215
Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, Nihira T, Kobayashi T et al (2012) Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 5:35. doi:10.1186/1756-6606-5-35
Byers B, Cord B, Nguyen HN, Schule B, Fenno L, Lee PC, Deisseroth K, Langston JW et al (2011) SNCA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS One 6(11):e26159. doi:10.1371/journal.pone.0026159
Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, McLean JR, Carrillo-Reid L et al (2012) Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med 4(141):141ra190. doi:10.1126/scitranslmed.3003985
Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C et al (2008) Disease-specific induced pluripotent stem cells. Cell 134(5):877–886. doi:10.1016/j.cell.2008.07.041
Zhang N, An MC, Montoro D, Ellerby LM (2010) Characterization of human Huntington’s disease cell model from induced pluripotent stem cells. PLoS Curr 2:RRN1193. doi:10.1371/currents.RRN1193
Consortium HDi (2012) Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell 11(2):264–278. doi:10.1016/j.stem.2012.04.027
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5(6):311–322. doi:10.1038/nrneurol.2009.54
Chae JI, Kim DW, Lee N, Jeon YJ, Jeon I, Kwon J, Kim J, Soh Y et al (2012) Quantitative proteomic analysis of induced pluripotent stem cells derived from a human Huntington’s disease patient. Biochem J 446(3):359–371. doi:10.1042/BJ20111495
Camnasio S, Delli Carri A, Lombardo A, Grad I, Mariotti C, Castucci A, Rozell B, Lo Riso P et al (2012) The first reported generation of several induced pluripotent stem cell lines from homozygous and heterozygous Huntington’s disease patients demonstrates mutation related enhanced lysosomal activity. Neurobiol Dis 46(1):41–51. doi:10.1016/j.nbd.2011.12.042
An MC, Zhang N, Scott G, Montoro D, Wittkop T, Mooney S, Melov S, Ellerby LM (2012) Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 11(2):253–263. doi:10.1016/j.stem.2012.04.026
Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H et al (2011) Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet 20(23):4530–4539. doi:10.1093/hmg/ddr394
Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S et al (2012) Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482(7384):216–220. doi:10.1038/nature10821
Koch P, Tamboli IY, Mertens J, Wunderlich P, Ladewig J, Stuber K, Esselmann H, Wiltfang J et al (2012) Presenilin-1 L166P mutant human pluripotent stem cell-derived neurons exhibit partial loss of gamma-secretase activity in endogenous amyloid-beta generation. Am J Pathol 180(6):2404–2416. doi:10.1016/j.ajpath.2012.02.012
Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ et al (2014) The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet 23(13):3523–3536. doi:10.1093/hmg/ddu064
Hossini AM, Megges M, Prigione A, Lichtner B, Toliat MR, Wruck W, Schroter F, Nuernberg P et al (2015) Induced pluripotent stem cell-derived neuronal cells from a sporadic Alzheimer’s disease donor as a model for investigating AD-associated gene regulatory networks. BMC Genomics 16:84. doi:10.1186/s12864-015-1262-5
Acknowledgments
AC Rego research is funded by FEDER funds through the Operational Programme Competitiveness Factors—COMPETE and national funds by ‘Fundação para a Ciência e a Tecnologia’ (FCT) from CNC.IBILI strategic project PEst-C/SAU/LA0001/2013-2014 and UID/NEU/04539/2013, and ‘Fundação Luso-Americana para o Desenvolvimento’ (FLAD) Life Science 2020, Portugal. C Lopes was a recipient of a PhD fellowship from FCT, Portugal, reference SFRH/BD/51192/2010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lopes, C., Rego, A.C. Revisiting Mitochondrial Function and Metabolism in Pluripotent Stem Cells: Where Do We Stand in Neurological Diseases?. Mol Neurobiol 54, 1858–1873 (2017). https://doi.org/10.1007/s12035-016-9714-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9714-8