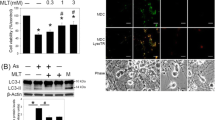
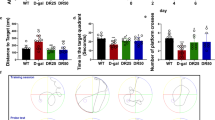
Abstract
Our previous investigation demonstrated that autophagy significantly reduced melamine-induced cell death in PC12 cells via inhibiting the excessive generation of ROS. In the present study, we further examine if rapamycin, used as an autophagy activator, can play a significant role in protecting neurons and alleviating the impairment of spatial cognition and hippocampal synaptic plasticity in melamine-treated rats. Male Wistar rats were divided into three groups: control, melamine-treated, and melamine-treated + rapamycin. The animal model was established by administering melamine at a dose of 300 mg/kg/day for 4 weeks. Rapamycin was intraperitoneally given at a dose of 1 mg/kg/day for 28 consecutive days. The Morris water maze test showed that spatial learning and reversal learning in melamine-treated rats were considerably damaged, whereas rapamycin significantly impeded the cognitive function impairment. Rapamycin efficiently alleviated the melamine-induced impairments of both long-term potentiation (LTP) and depotentiation, which were damaged in melamine rats. Rapamycin further increased the expression level of autophagy markers, which were significantly enhanced in melamine rats. Moreover, rapamycin noticeably decreased the reactive oxygen species level, while the superoxide dismutase activity was remarkably increased by rapamycin in melamine rats. Malondialdehyde assay exhibited that rapamycin prominently reduced the malondialdehyde (MDA) level of hippocampal neurons in melamine-treated rats. In addition, rapamycin significantly decreased the caspase-3 activity, which was elevated by melamine. Consequently, our results suggest that regulating autophagy may become a new targeted therapy to relieve the damage induced by melamine.








Similar content being viewed by others
References
Han YG, Liu SC, Zhang T, Yang Z (2011) Induction of apoptosis by melamine in differentiated PC12 cells. Cell Mol Neurobiol 31(1):65–71. doi:10.1007/s10571-010-9554-4
Wang Y, Liu F, Wei Y, Liu D (2011) The effect of exogenous melamine on rat hippocampal neurons. Toxicol Ind Health 27:571–576
Yang J, An L, Yao Y, Yang Z, Zhang T (2011) Melamine impairs spatial cognition and hippocampal synaptic plasticity by presynaptic inhibition of glutamatergic transmission in infant rats. Toxicology 289(2–3):167–174. doi:10.1016/j.tox.2011.08.011
Yang JJ, Tian YT, Yang Z, Zhang T (2010) Effect of melamine on potassium currents in rat hippocampal CA1 neurons. Toxicol in Vitro 24(2):397–403. doi:10.1016/j.tiv.2009.10.019
Yang JJ, Yang Z, Zhang T (2010) Action potential changes associated with impairment of functional properties of sodium channels in hippocampal neurons induced by melamine. Toxicol Lett 198(2):171–176
Wu YT, Huang CM, Lin CC, Ho WA, Lin LC, Chiu TF, Tarng DC, Lin CH et al (2009) Determination of melamine in rat plasma, liver, kidney, spleen, bladder and brain by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1216(44):7595–7601
An L, Li Z, Yang Z, Zhang T (2011) Cognitive deficits induced by melamine in rats. Toxicol Lett 206(3):276–280. doi:10.1016/j.toxlet.2011.08.009
An L, Yang Z, Zhang T (2013) Melamine induced spatial cognitive deficits associated with impairments of hippocampal long-term depression and cholinergic system in Wistar rats. Neurobiol Learn Mem 100:18–24. doi:10.1016/j.nlm.2012.12.003
Ma C, Kang H, Liu Q, Zhu R, Cao Z (2011) Insight into potential toxicity mechanisms of melamine: an in silico study. Toxicology 283(2–3):96–100. doi:10.1016/j.tox.2011.02.009
Guo C, He Z, Wen L, Zhu L, Lu Y, Deng S, Yang Y, Wei Q et al (2012) Cytoprotective effect of trolox against oxidative damage and apoptosis in the NRK-52e cells induced by melamine. Cell Biol Int 36(2):183–188. doi:10.1042/CBI20110036
An L, Li Z, Zhang T (2014) Reversible effects of vitamins C and E combination on oxidative stress-induced apoptosis in melamine-treated PC12 cells. Free Radic Res 48(2):239–250. doi:10.3109/10715762.2013.861598
Kemp A, Manahan-Vaughan D (2007) Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci 30(3):111–118. doi:10.1016/j.tins.2007.01.002
Kulla A, Reymann KG, Manahan-Vaughan D (1999) Time-dependent induction of depotentiation in the dentate gyrus of freely moving rats: involvement of group 2 metabotropic glutamate receptors. Eur J Neurosci 11(11):3864–3872. doi:10.1046/j.1460-9568.1999.00807.x
Huang YZ, Rothwell JC, Lu CS, Chuang WL, Lin WY, Chen RS (2010) Reversal of plasticity-like effects in the human motor cortex. J Physiol 588(Pt 19):3683–3693. doi:10.1113/jphysiol.2010.191361
Citri A, Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33(1):18–41. doi:10.1038/sj.npp.1301559
Ehrlich I, Klein M, Rumpel S, Malinow R (2007) PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A 104(10):4176–4181. doi:10.1073/pnas.0609307104
Li J, Kim SG, Blenis J (2014) Rapamycin: one drug, many effects. Cell Metab 19(3):373–379. doi:10.1016/j.cmet.2014.01.001
Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R et al (2008) Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest 118(2):777–788. doi:10.1172/JCI32806
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 285(17):13107–13120. doi:10.1074/jbc.M110.100420
Lipton JO, Sahin M (2014) The neurology of mTOR. Neuron 84(2):275–291. doi:10.1016/j.neuron.2014.09.034
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221(1):3–12. doi:10.1002/path.2697
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115(10):2679–2688. doi:10.1172/JCI26390
Carloni S, Buonocore G, Balduini W (2008) Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis 32(3):329–339. doi:10.1016/j.nbd.2008.07.022
Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH (2010) Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J Neurosci 30(45):14946–14954. doi:10.1523/JNEUROSCI.4305-10.2010
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. doi:10.1038/nature05292
Knecht E, Criado-Garcia O, Aguado C, Gayarre J, Duran-Trio L, Garcia-Cabrero AM, Vernia S, San Millan B et al (2012) Malin knockout mice support a primary role of autophagy in the pathogenesis of Lafora disease. Autophagy 8(4):701–703. doi:10.4161/auto.19522
Li L, Ishdorj G, Gibson SB (2012) Reactive oxygen species regulation of autophagy in cancer: implications for cancer treatment. Free Radic Biol Med 53(7):1399–1410. doi:10.1016/j.freeradbiomed.2012.07.011
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11(4):777–790. doi:10.1089/ARS.2008.2270
Wang H, Gao N, Li Z, Yang Z, Zhang T (2015) Autophagy alleviates melamine-induced cell death in PC12 cells via decreasing ROS level. Mol Neurobiol. doi:10.1007/s12035-014-9073-2
Han G, An L, Yang B, Si L, Zhang T (2014) Nicotine-induced impairments of spatial cognition and long-term potentiation in adolescent male rats. Hum Exp Toxicol 33(2):203–213. doi:10.1177/0960327113494902
An L, Yang Z, Zhang T (2013) Imbalanced synaptic plasticity induced spatial cognition impairment in male offspring rats treated with chronic prenatal ethanol exposure. Alcohol Clin Exp Res 37(5):763–770
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E et al (2014) Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83(5):1131–1143. doi:10.1016/j.neuron.2014.07.040
An L, Zhang T (2014) Vitamins C and E reverse melamine-induced deficits in spatial cognition and hippocampal synaptic plasticity in rats. Neurotoxicology 44:132–139. doi:10.1016/j.neuro.2014.06.009
Yu M, Zhang Y, Chen X, Zhang T (2015) Antidepressant-like effects and possible mechanisms of amantadine on cognitive and synaptic deficits in depression rats. Stress. doi:10.3109/10253890.2015.1108302
Liu C, Xu X, Gao J, Zhang T, Yang Z (2015) Hydrogen sulfide prevents synaptic plasticity from VD-induced damage via Akt/GSK-3beta pathway and notch signaling pathway in rats. Mol Neurobiol. doi:10.1007/s12035-015-9324-x
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858. doi:10.1038/nprot.2006.116
An L, Li Z, Yang Z, Zhang T (2012) Melamine induced cognitive impairment associated with oxidative damage in rat’s hippocampus. Pharmacol Biochem Behav 102(2):196–202. doi:10.1016/j.pbb.2012.04.009
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361(6407):31–39. doi:10.1038/361031a0
Qi Y, Hu NW, Rowan MJ (2013) Switching off LTP: mGlu and NMDA receptor-dependent novelty exploration-induced depotentiation in the rat hippocampus. Cereb Cortex 23(4):932–939. doi:10.1093/cercor/bhs086
Li B, Otsu Y, Murphy TH, Raymond LA (2003) Developmental decrease in NMDA receptor desensitization associated with shift to synapse and interaction with postsynaptic density-95. J Neurosci 23(35):11244–11254
Yao H, Zhao D, Khan SH, Yang L (2013) Role of autophagy in prion protein-induced neurodegenerative diseases. Acta Biochim Biophys Sin 45(6):494–502. doi:10.1093/abbs/gmt022
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873. doi:10.1101/gad.1599207
McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT et al (2011) Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 364(17):1595–1606. doi:10.1056/NEJMoa1100391
Sahin M (2012) Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Curr Opin Neurobiol 22(5):895–901. doi:10.1016/j.conb.2012.04.008
Franz DN (2013) Everolimus in the treatment of subependymal giant cell astrocytomas, angiomyolipomas, and pulmonary and skin lesions associated with tuberous sclerosis complex. Biologics : targets & therapy 7:211–221. doi:10.2147/BTT.S25095
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ (2008) Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med 14(8):843–848. doi:10.1038/nm1788
Caccamo A, De Pinto V, Messina A, Branca C, Oddo S (2014) Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci 34(23):7988–7998. doi:10.1523/JNEUROSCI.0777-14.2014
Pryor WM, Biagioli M, Shahani N, Swarnkar S, Huang WC, Page DT, MacDonald ME, Subramaniam S (2014) Huntingtin promotes mTORC1 signaling in the pathogenesis of Huntington’s disease. Sci Signal 7(349), ra103. doi:10.1126/scisignal.2005633
Nikoletopoulou V, Papandreou ME, Tavernarakis N (2015) Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ 22(3):398–407. doi:10.1038/cdd.2014.204
Li L, Zhang Q, Tan J, Fang Y, An X, Chen B (2014) Autophagy and hippocampal neuronal injury. Sleep Breath = Schlaf & Atmung 18(2):243–249. doi:10.1007/s11325-013-0930-4
Chen Q, Niu Y, Zhang R, Guo H, Gao Y, Li Y, Liu R (2010) The toxic influence of paraquat on hippocampus of mice: involvement of oxidative stress. Neurotoxicology 31(3):310–316. doi:10.1016/j.neuro.2010.02.006
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. doi:10.1038/nature04723
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36(6):585–595. doi:10.1038/ng1362
Milisav I, Suput D, Ribaric S (2015) Unfolded protein response and macroautophagy in Alzheimer’s, Parkinson’s and prion diseases. Molecules 20(12):22718–22756. doi:10.3390/molecules201219865
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. doi:10.1016/j.cell.2011.10.026
Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC (2009) Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 16(1):46–56. doi:10.1038/cdd.2008.110
Fagundes LS, Fleck AD, Zanchi AC, Saldiva PH, Rhoden CR (2015) Direct contact with particulate matter increases oxidative stress in different brain structures. Inhalation toxicology. 1–6. doi:10.3109/08958378.2015.1060278
Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y (2008) Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med 45(11):1542–1550. doi:10.1016/j.freeradbiomed.2008.08.030
Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8(1):3–5. doi:10.1089/rej.2005.8.3
Yuan Y, Zhang X, Zheng Y, Chen Z (2015) Regulation of mitophagy in ischemic brain injury. Neurosci Bull 31(4):395–406. doi:10.1007/s12264-015-1544-6
Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, Yongvanit P, Kawanishi S et al (2015) Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci 16(1):193–217. doi:10.3390/ijms16010193
Kamalinia G, Khodagholi F, Atyabi F, Amini M, Shaerzadeh F, Sharifzadeh M, Dinarvand R (2013) Enhanced brain delivery of deferasirox-lactoferrin conjugates for iron chelation therapy in neurodegenerative disorders: in vitro and in vivo studies. Mol Pharm 10(12):4418–4431. doi:10.1021/mp4002014
Riedl SJ, Shi Y (2004) Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 5(11):897–907. doi:10.1038/nrm1496
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM (2002) A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A 99(1):467–472. doi:10.1073/pnas.012605299
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (11232005, 31171053), and 111 Project (B08011).
Authors’ Contributions
JF, HW, and TZ conceived and designed the experiment, JF, HW, JG, MY, RW, and ZY performed the experiments and analyzed the data; and JF and TZ wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they no competing interests.
Additional information
Jingxuan Fu and Hui Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fu, J., Wang, H., Gao, J. et al. Rapamycin Effectively Impedes Melamine-Induced Impairments of Cognition and Synaptic Plasticity in Wistar Rats. Mol Neurobiol 54, 819–832 (2017). https://doi.org/10.1007/s12035-016-9687-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9687-7