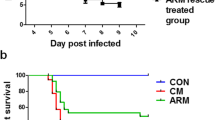
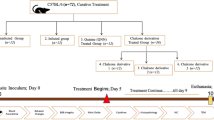
Abstract
Cerebral malaria (CM) is a life-threatening complication of Plasmodium falciparum infection, which can result in long-term cognitive and behavioral deficits despite successful anti-malarial therapy. Due to the substantial social and economic burden of CM, the development of adjuvant therapies is a scientific goal of highest priority. Apart from vascular and immune responses, changes in glutamate system have been reported in CM pathogenesis suggesting a potential therapeutic target. Based on that, we hypothesized that interventions in the glutamatergic system induced by blockage of N-methyl-D-aspartate (NMDA) receptors could attenuate experimental CM long-term cognitive and behavioral outcomes. Before the development of evident CM signs, susceptible mice infected with Plasmodium berghei ANKA (PbA) strain were initiated on treatment with dizocilpine maleate (MK801, 0.5 mg/kg), a noncompetitive NMDA receptor antagonist. On day 5 post-infection, mice were treated orally with a 10-day course chloroquine (CQ, 30 mg/kg). Control mice also received saline, CQ or MK801 + CQ therapy. After 10 days of cessation of CQ treatment, magnetic resonance images (MRI), behavioral and immunological assays were performed. Indeed, MK801 combined with CQ prevented long-term memory impairment and depressive-like behavior following successful PbA infection resolution. In addition, MK801 also modulated the immune system by promoting a balance of TH1/TH2 response and upregulating neurotrophic factors levels in the frontal cortex and hippocampus. Moreover, hippocampus abnormalities observed by MRI were partially prevented by MK801 treatment. Our results indicate that NMDA receptor antagonists can be neuroprotective in CM and could be a valuable adjuvant strategy for the management of the long-term impairment observed in CM.









Similar content being viewed by others
References
de Souza JB, Hafalla JC, Riley EM, Couper KN (2010) Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137(5):755–772. doi:10.1017/S0031182009991715S0031182009991715
Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, John CC (2007) Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics 119(2):e360–e366. doi:10.1542/peds.2006-2027
Carter JA, Mung’ala-Odera V, Neville BG, Murira G, Mturi N, Musumba C, Newton CR (2005) Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry 76(4):476–481. doi:10.1136/jnnp.2004.043893
John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, Wu B, Boivin MJ (2008) Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 122(1):e92–e99. doi:10.1542/peds.2007-3709
Fernando SD, Rodrigo C, Rajapakse S (2010) The ‘hidden’ burden of malaria: cognitive impairment following infection. Malar J 9:366. doi:10.1186/1475-2875-9-366
Varney NR, Roberts RJ, Springer JA, Connell SK, Wood PS (1997) Neuropsychiatric sequelae of cerebral malaria in Vietnam veterans. J Nerv Ment Dis 185(11):695–703
Dugbartey AT, Dugbartey MT, Apedo MY (1998) Delayed neuropsychiatric effects of malaria in Ghana. J Nerv Ment Dis 186(3):183–186
de Miranda AS, Lacerda-Queiroz N, de Carvalho VM, Rodrigues DH, Rachid MA, Quevedo J, Teixeira AL (2011) Anxiety-like behavior and proinflammatory cytokine levels in the brain of C57BL/6 mice infected with Plasmodium berghei (strain ANKA). Neurosci Lett 491(3):202–206. doi:10.1016/j.neulet.2011.01.038S0304-3940(11)00074-7
John CC, Panoskaltsis-Mortari A, Opoka RO, Park GS, Orchard PJ, Jurek AM, Idro R, Byarugaba J et al (2008) Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. AmJTrop Med Hyg 78(2):198–205
Idro R, Kakooza-Mwesige A, Balyejjussa S, Mirembe G, Mugasha C, Tugumisirize J, Byarugaba J (2010) Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res Notes 3:104. doi:10.1186/1756-0500-3-104
van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE (2006) A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol 22(11):503–508. doi:10.1016/j.pt.2006.09.002
Hunt NH, Golenser J, Chan-Ling T, Parekh S, Rae C, Potter S, Medana IM et al (2006) Immunopathogenesis of cerebral malaria. Int J Parasitol 36(5):569–582. doi:10.1016/j.ijpara.2006.02.016
Rae C, McQuillan JA, Parekh SB, Bubb WA, Weiser S, Balcar VJ, Hansen AM, Ball HJ et al (2004) Brain gene expression, metabolism, and bioenergetics: interrelationships in murine models of cerebral and noncerebral malaria. FASEB J 18(3):499–510. doi:10.1096/fj.03-0543com18/3/499
Parekh SB, Bubb WA, Hunt NH, Rae C (2006) Brain metabolic markers reflect susceptibility status in cytokine gene knockout mice with murine cerebral malaria. Int J Parasitol 36(13):1409–1418. doi:10.1016/j.ijpara.2006.07.004
Miranda AS, Vieira LB, Lacerda-Queiroz N, Souza AH, Rodrigues DH, Vilela MC, Gomez MV, Machado FS et al (2010) Increased levels of glutamate in the central nervous system are associated with behavioral symptoms in experimental malaria. Braz J Med Biol Res 43(12):1173–1177
Meldrum BS (2000) Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130(4S Suppl):1007S–1015S
Schauwecker PE (2010) Neuroprotection by glutamate receptor antagonists against seizure-induced excitotoxic cell death in the aging brain. Exp Neurol 224(1):207–218. doi:10.1016/j.expneurol.2010.03.013
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8):856–864. doi:10.1001/archpsyc.63.8.856
Engin E, Treit D, Dickson CT (2009) Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience 161(2):359–369. doi:10.1016/j.neuroscience.2009.03.038S0306-4522(09)00372-8
Bano D, Nicotera P (2007) Ca2+ signals and neuronal death in brain ischemia. Stroke 38(2 Suppl):674–676. doi:10.1161/01.STR.0000256294.46009.29
Wang Y, Qin ZH (2010) Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 15(11):1382–1402. doi:10.1007/s10495-010-0481-0
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460(2):525–542. doi:10.1007/s00424-010-0809-1
Yan D, Yamasaki M, Straub C, Watanabe M, Tomita S (2013) Homeostatic control of synaptic transmission by distinct glutamate receptors. Neuron 78(4):687–699. doi:10.1016/j.neuron.2013.02.031S0896-6273(13)00189-X
Tayeb HO, Yang HD, Price BH, Tarazi FI (2012) Pharmacotherapies for Alzheimer’s disease: beyond cholinesterase inhibitors. Pharmacol Ther 134(1):8–25. doi:10.1016/j.pharmthera.2011.12.002
Bakiri Y, Hamilton NB, Karadottir R, Attwell D (2008) Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia 56(2):233–240. doi:10.1002/glia.20608
Cassol-Jr OJ, Comim CM, Constantino LS, Rosa DV, Mango LA, Stertz L, Kapczinski F, Romano-Silva MA et al (2011) Acute low dose of MK-801 prevents memory deficits without altering hippocampal DARPP-32 expression and BDNF levels in sepsis survivor rats. J Neuroimmunol 230(1–2):48–51. doi:10.1016/j.jneuroim.2010.08.026
Esposito E, Paterniti I, Mazzon E, Genovese T, Galuppo M, Meli R, Bramanti P, Cuzzocrea S (2011) MK801 attenuates secondary injury in a mouse experimental compression model of spinal cord trauma. BMC Neurosci 12:31. doi:10.1186/1471-2202-12-31
Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F (2012) New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol 73(4):504–517. doi:10.1111/j.1365-2125.2011.04134.x
Picot S, Bienvenu AL, Konate S, Sissoko S, Barry A, Diarra E, Bamba K, Djimde A et al (2009) Safety of epoietin beta-quinine drug combination in children with cerebral malaria in Mali. Malar J 8:169. doi:10.1186/1475-2875-8-1691475-2875-8-169
Casals-Pascual C, Idro R, Gicheru N, Gwer S, Kitsao B, Gitau E, Mwakesi R, Roberts DJ et al (2008) High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc Natl Acad Sci U S A 105(7):2634–2639. doi:10.1073/pnas.0709715105
Reis PA, Estato V, da Silva TI, d’Avila JC, Siqueira LD, Assis EF, Bozza PT, Bozza FA et al (2012) Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog 8(12):e1003099. doi:10.1371/journal.ppat.1003099PPATHOGENS-D-12-01593
Dai M, Freeman B, Shikani HJ, Bruno FP, Collado JE, Macias R, Reznik SE, Davies P et al (2012) Altered regulation of Akt signaling with murine cerebral malaria, effects on long-term neuro-cognitive function, restoration with lithium treatment. PLoS One 7(10):e44117. doi:10.1371/journal.pone.0044117PONE-D-11-25736
Grau GE, Piguet PF, Engers HD, Louis JA, Vassalli P, Lambert PH (1986) L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol 137(7):2348–2354
Carroll RW, Wainwright MS, Kim KY, Kidambi T, Gomez ND, Taylor T, Haldar K (2010) A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One 5(10). doi:10.1371/journal.pone.0013124e13124
Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE (1997) Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8(10):711–713
Clemmer L, Martins YC, Zanini GM, Frangos JA, Carvalho LJ (2011) Artemether and artesunate show the highest efficacies in rescuing mice with late-stage cerebral malaria and rapidly decrease leukocyte accumulation in the brain. Antimicrob Agents Chemother 55(4):1383–1390. doi:10.1128/AAC.01277-10
Dai M, Reznik SE, Spray DC, Weiss LM, Tanowitz HB, Gulinello M, Desruisseaux MS (2010) Persistent cognitive and motor deficits after successful antimalarial treatment in murine cerebral malaria. Microbes Infect 12(14–15):1198–1207. doi:10.1016/j.micinf.2010.08.006
Romano-Silva MA, Ribeiro-Santos R, Ribeiro AM, Gomez MV, Diniz CR, Cordeiro MN, Brammer MJ (1993) Rat cortical synaptosomes have more than one mechanism for Ca2+ entry linked to rapid glutamate release: studies using the Phoneutria nigriventer toxin PhTX2 and potassium depolarization. Biochem J 296(Pt 2):313–319
Dunkley PR, Heath JW, Harrison SM, Jarvie PE, Glenfield PJ, Rostas JA (1988) A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Res 441(1–2):59–71
Nicholls DG, Sihra TS, Sanchez-Prieto J (1987) Calcium-dependent and -independent release of glutamate from synaptosomes monitored by continuous fluorometry. J Neurochem 49(1):50–57
Reis PA, Comim CM, Hermani F, Silva B, Barichello T, Portella AC, Gomes FC, Sab IM et al (2010) Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog 6(6):e1000963. doi:10.1371/journal.ppat.1000963
Haring M, Grieb M, Monory K, Lutz B, Moreira FA (2013) Cannabinoid CB(1) receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology 65:83–89. doi:10.1016/j.neuropharm.2012.09.002S0028-3908(12)00474-1
Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92(2):180–185
File SE (2001) Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res 125(1–2):151–157
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–328. doi:10.1038/nprot.2007.44
Brant F, Miranda AS, Esper L, Rodrigues DH, Kangussu LM, Bonaventura D, Soriani FM, Pinho V et al (2014) Role of the aryl hydrocarbon receptor in the immune response profile and development of pathology during Plasmodium berghei Anka infection. Infect Immun 82(8):3127–3140. doi:10.1128/IAI.01733-14
Desruisseaux MS, Gulinello M, Smith DN, Lee SC, Tsuji M, Weiss LM, Spray DC, Tanowitz HB (2008) Cognitive dysfunction in mice infected with Plasmodium berghei strain ANKA. J Infect Dis 197(11):1621–1627. doi:10.1086/587908
Lackner P, Beer R, Heussler V, Goebel G, Rudzki D, Helbok R, Tannich E, Schmutzhard E (2006) Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol Appl Neurobiol 32(2):177–188. doi:10.1111/j.1365-2990.2006.00706.x
Roenker NL, Gudelsky GA, Ahlbrand R, Horn PS, Richtand NM (2012) Evidence for involvement of nitric oxide and GABA(B) receptors in MK-801-stimulated release of glutamate in rat prefrontal cortex. Neuropharmacology 63(4):575–581. doi:10.1016/j.neuropharm.2012.04.032S0028-3908(12)00168-2
Angelucci F, Brene S, Mathe AA (2005) BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry 10(4):345–352. doi:10.1038/sj.mp.4001637
Schmidt HD, Duman RS (2007) The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 18(5–6):391–418. doi:10.1097/FBP.0b013e3282ee2aa800008877-200709000-00008
Conner JM, Franks KM, Titterness AK, Russell K, Merrill DA, Christie BR, Sejnowski TJ, Tuszynski MH (2009) NGF is essential for hippocampal plasticity and learning. J Neurosci 29(35):10883–10889. doi:10.1523/JNEUROSCI.2594-09.200929/35/10883
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ (2007) Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12(7):656–670. doi:10.1038/sj.mp.4001957
Potchen MJ, Kampondeni SD, Seydel KB, Birbeck GL, Hammond CA, Bradley WG, DeMarco JK, Glover SJ et al (2012) Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. AJNR Am J Neuroradiol 33(9):1740–1746. doi:10.3174/ajnr.A3035ajnr.A3035
Penet MF, Viola A, Confort-Gouny S, Le Fur Y, Duhamel G, Kober F, Ibarrola D et al (2005) Imaging experimental cerebral malaria in vivo: significant role of ischemic brain edema. J Neurosci 25(32):7352–7358. doi:10.1523/JNEUROSCI.1002-05.2005
Jelicks LA, Lisanti MP, Machado FS, Weiss LM, Tanowitz HB, Desruisseaux MS (2013) Imaging of small-animal models of infectious diseases. Am J Pathol 182(2):296–304. doi:10.1016/j.ajpath.2012.09.026S0002-9440(12)00822-X
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354
Yang C, Hong T, Shen J, Ding J, Dai XW, Zhou ZQ, Yang JJ (2013) Ketamine exerts antidepressant effects and reduces IL-1beta and IL-6 levels in rat prefrontal cortex and hippocampus. Exp Ther Med 5(4):1093–1096. doi:10.3892/etm.2013.930etm-05-04-1093
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475(7354):91–95. doi:10.1038/nature10130nature10130
McCusker RH, Kelley KW (2013) Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol 216(Pt 1):84–98. doi:10.1242/jeb.073411
York JM, Blevins NA, Baynard T, Freund GG (2012) Mouse testing methods in psychoneuroimmunology: an overview of how to measure sickness, depressive/anxietal, cognitive, and physical activity behaviors. Methods Mol Biol 934:243–276. doi:10.1007/978-1-62703-071-7_13
Allahtavakoli M, Shabanzadeh A, Roohbakhsh A, Pourshanazari A (2007) Combination therapy of rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, and NMDA receptor antagonist (MK-801) on experimental embolic stroke in rats. Basic Clin Pharmacol Toxicol 101(5):309–314. doi:10.1111/j.1742-7843.2007.00127.x
McAfoose J, Baune BT (2009) Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 33(3):355–366. doi:10.1016/j.neubiorev.2008.10.005S0149-7634(08)00183-8
Rocha NP, Teixeira AL, Coelho FM, Caramelli P, Guimaraes HC, Barbosa IG, da Silva TA, Mukhamedyarov MA et al (2012) Peripheral blood mono-nuclear cells derived from Alzheimer’s disease patients show elevated baseline levels of secreted cytokines but resist stimulation with beta-amyloid peptide. Mol Cell Neurosci 49(1):77–84. doi:10.1016/j.mcn.2011.09.005S1044-7431(11)00213-2
Linares M, Marin-Garcia P, Perez-Benavente S, Sanchez-Nogueiro J, Puyet A, Bautista JM, Diez A (2013) Brain-derived neurotrophic factor and the course of experimental cerebral malaria. Brain Res 1490:210–224. doi:10.1016/j.brainres.2012.10.040S0006-8993(12)01697-6
Barichello T, Dos Santos I, Savi GD, Simoes LR, Generoso JS, Comim CM, Sachs D, Teixeira AL et al (2010) Depressive-like-behavior and proinflamatory interleukine levels in the brain of rats submitted to pneumococcal meningitis. Brain Res Bull 82(5–6):243–246. doi:10.1016/j.brainresbull.2010.04.015S0361-9230(10)00096-1
de Souza JB, Riley EM (2002) Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect 4(3):291–300
Kossodo S, Monso C, Juillard P, Velu T, Goldman M, Grau GE (1997) Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91(4):536–540
Hunt NH, Grau GE (2003) Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol 24(9):491–499
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G et al (2001) CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci 4(7):702–710. doi:10.1038/8949089490
Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, Chen SY, Raung SL, Lai CY (2012) Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-alpha signaling and contributes to neuronal death. Glia 60(3):487–501. doi:10.1002/glia.22282
Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC (2013) IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. doi:10.1111/jnc.12263
Jander S, Schroeter M, Stoll G (2000) Role of NMDA receptor signaling in the regulation of inflammatory gene expression after focal brain ischemia. J Neuroimmunol 109(2):181–187
Al-Amin H, Sarkis R, Atweh S, Jabbur S, Saade N (2011) Chronic dizocilpine or apomorphine and development of neuropathy in two animal models II: effects on brain cytokines and neurotrophins. Exp Neurol 228(1):30–40. doi:10.1016/j.expneurol.2010.11.005S0014-4886(10)00406-1
Liu CH, Cherng CH, Lin SL, Yeh CC, Wu CT, Tai YH, Wong CS (2011) N-methyl-D-aspartate receptor antagonist MK-801 suppresses glial pro-inflammatory cytokine expression in morphine-tolerant rats. Pharmacol Biochem Behav 99(3):371–380. doi:10.1016/j.pbb.2011.05.016S0091-3057(11)00158-4
Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M (2006) Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31(1):149–160. doi:10.1016/j.mcn.2005.10.006
Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J (2010) Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 207(5):1067–1080. doi:10.1084/jem.20091419jem.20091419
Dobbertin A, Gervais A, Glowinski J, Mallat M (2000) Activation of ionotropic glutamate receptors reduces the production of transforming growth factor-beta2 by developing neurons. Eur J Neurosci 12(12):4589–4593
Wilson KD, Stutz SJ, Ochoa LF, Valbuena GA, Cravens PD, Dineley KT, Vargas G, Stephens R (2016) Behavioural and neurological symptoms accompanied by cellular neuroinflammation in IL-10-deficient mice infected with Plasmodium chabaudi. Malar J 15(1):428. doi:10.1186/s12936-016-1477-1
Ransohoff RM (2009) Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31(5):711–721. doi:10.1016/j.immuni.2009.09.010S1074-7613(09)00422-1
de Haas AH, van Weering HR, de Jong EK, Boddeke HW, Biber KP (2007) Neuronal chemokines: versatile messengers in central nervous system cell interaction. Mol Neurobiol 36(2):137–151. doi:10.1007/s12035-007-0036-8
Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S (2005) Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem 95(4):1023–1034. doi:10.1111/j.1471-4159.2005.03431.x
Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Nahon JL, Rostene W et al (2007) The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J Neurochem 102(4):1175–1183. doi:10.1111/j.1471-4159.2007.04639.x
Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, Adjei AA, Gyasi RK et al (2007) Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J 6:147. doi:10.1186/1475-2875-6-147
Lacerda-Queiroz N, Rodrigues DH, Vilela MC, Miranda AS, Amaral DC, Camargos ER, Carvalho LJ, Howe CL et al (2010) Inflammatory changes in the central nervous system are associated with behavioral impairment in Plasmodium berghei (strain ANKA)-infected mice. Exp Parasitol 125(3):271–278. doi:10.1016/j.exppara.2010.02.002
Wilson NO, Jain V, Roberts CE, Lucchi N, Joel PK, Singh MP, Nagpal AC, Dash AP et al (2011) CXCL4 and CXCL10 predict risk of fatal cerebral malaria. Dis Markers 30(1):39–49. doi:10.3233/DMA-2011-0763D67R370H3U3318JK
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N et al (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477(7362):90–94. doi:10.1038/nature10357nature10357
Stevenson NJ, Addley MR, Ryan EJ, Boyd CR, Carroll HP, Paunovic V, Bursill CA, Miller HC et al (2009) CCL11 blocks IL-4 and GM-CSF signaling in hematopoietic cells and hinders dendritic cell differentiation via suppressor of cytokine signaling expression. J Leukoc Biol 85(2):289–297. doi:10.1189/jlb.0708394jlb.0708394
Comim CM, Reis PA, Frutuoso VS, Fries GR, Fraga DB, Kapczinski F, Zugno AI, Barichello T et al (2012) Effects of experimental cerebral malaria in memory, brain-derived neurotrophic factor and acetylcholinesterase activity [correction for activity] in the hippocampus of survivor mice. Neurosci Lett 523(2):104–107. doi:10.1016/j.neulet.2012.06.051S0304-3940(12)00859-2
Michel TM, Frangou S, Camara S, Thiemeyer D, Jecel J, Tatschner T, Zoechling R, Grunblatt E (2008) Altered glial cell line-derived neurotrophic factor (GDNF) concentrations in the brain of patients with depressive disorder: a comparative post-mortem study. Eur Psychiatry 23(6):413–420. doi:10.1016/j.eurpsy.2008.06.001S0924-9338(08)01546-0
Matsuki H, Shirayama Y, Hashimoto K, Tanaka A, Minabe Y (2001) Effects of age and gender on the expression of brain-derived neurotrophic factor mRNA in rat retrosplenial cortex following administration of dizocilpine. Neuropsychopharmacology 25(2):258–266. doi:10.1016/S0893-133X(00)00246-3
Linden AM, Vaisanen J, Lakso M, Nawa H, Wong G, Castren E (2000) Expression of neurotrophins BDNF and NT-3, and their receptors in rat brain after administration of antipsychotic and psychotrophic agents. J Mol Neurosci 14(1–2):27–37. doi:10.1385/JMN:14:1-2:027
Lu B, Nagappan G, Guan X, Nathan PJ, Wren P (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14(6):401–416. doi:10.1038/nrn3505nrn3505
Pertusa M, Garcia-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, Cristofol R, Sarkis C et al (2008) Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging 29(9):1366–1379. doi:10.1016/j.neurobiolaging.2007.02.026
Ji C, Song C, Zuo P (2011) The mechanism of memory impairment induced by Abeta chronic administration involves imbalance between cytokines and neurotrophins in the rat hippocampus. Curr Alzheimer Res 8(4):410–420
Kuno R, Yoshida Y, Nitta A, Nabeshima T, Wang J, Sonobe Y, Kawanokuchi J, Takeuchi H et al (2006) The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res 1116:12–18. doi:10.1016/j.brainres.2006.07.120
Takei Y, Laskey R (2008) Interpreting crosstalk between TNF-alpha and NGF: potential implications for disease. Trends Mol Med 14(9):381–388. doi:10.1016/j.molmed.2008.07.002S1471-4914(08)00147-0
Li SJ, Liu W, Wang JL, Zhang Y, Zhao DJ, Wang TJ, Li YY (2014) The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur Rev Med Pharmacol Sci 18(6):905–909
Miranda AS, Brant F, Campos AC, Vieira LB, Rocha NP, Cisalpino D, Binda NS, Rodrigues DH et al (2015) Evidence for the contribution of adult neurogenesis and hippocampal cell death in experimental cerebral malaria cognitive outcome. Neuroscience 284:920–933. doi:10.1016/j.neuroscience.2014.10.062
Acknowledgments
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Rede Instituto Brasileiro de Neurociência (IBNet/FINEP), Brazil.
Author’s contribution
ASM participated in the experimental design, carried out behavioral and immunological assays, data analysis, and drafted the manuscript. FB participated in the experimental design, carried out behavioral and immunological assays, and revised the manuscript. LBV and FMR carried out glutamate release assays, data analysis, and revised the manuscript. NPR and ELMV performed immunological assays, data analysis, and revised the manuscript. PMOP performed immunological assays and revised the manuscript. GHSR and MFDM performed MRI analysis. RMR and MMT were responsible for interpretation of data and revised and edited the manuscript. FSM and MAR participated in the design and coordination of the study. ALT designed the study and was responsible for the interpretation of experiments and editing the manuscript. All authors have read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest. Dr. Ransohoff is an employee of Biogen.
Electronic supplementary material
Figure S1
Study experimental design. Briefly, C57BL/6 mice were intraperitoneally infected with 106 parasitized erythrocytes. On day 3 post-infection, a group initiated MK801 (i.p. 0.5 mg/kg) treatment, which was continued until the end of chloroquine (CQ) therapy. All mice were treated orally with a 10-day course of CQ (30 mg/kg) starting at day 5 post-infection. As controls, non-infected animals received the same volume of saline, CQ or CQ + MK801. Ten days after cessation of CQ treatment, cognitive, behavioral, and magnetic resonance images analysis were performed. Moreover, brains were also harvested for immunological and histopathological assays. (GIF 82 kb)
Figure S2
Representative flow cytometry graphs of microglia F4/80+ CD11b+ expressing CD45 and IL10 from brain cells of C57/Bl6 mice. Cells from brain tissue from C57/Bl6 mice treated with MK801 (a) were stained and analyzed using flow cytometry as described in Materials and Methods. Flow cytometry dot-plots show the total cells (b). After selecting the cells double positive for F4/80 and CD11b (Q2 in B), CD45+ (c) was analyzed in different regions (CD45High and CD45Low) expressing IL-10 cytokine. The cytokine expression was analyzed in CD45High in F4/80+CD11b+ (D) or CD45Low in F4/80+CD11b+ (e). For all cytokine analysis, control isotypes were used for determination of cytokine expression using histogram graphs. (GIF 274 kb)
Figure S3
Effect of MK801 on hippocampus glutamate release following Plasmodium berghei ANKA (PbA) infection resolution by chloroquine (CQ) therapy. Ten days after cessation of CQ therapy, all animals were culled, hippocampus was harvested, synaptosomes were prepared, and glutamate release in the hippocampus was measured by spectrofluorometer. Results are expressed as mean ± SEM and are representative of two independent experiments (n = 5 per group). Asterisk(s) indicate statistical differences where *p < 0.05, **p < 0.01, ***p < 0.001. (GIF 17 kb)
Figure S4
Effect of MK801 in long-term aversive memory following Plasmodium berghei ANKA (PbA) infection resolution by chloroquine (CQ) therapy. From 10 days after cessation of CQ treatment, all mice were submitted to the step-down inhibitory avoidance test. No significant difference in the step-down latency was found in the training session or in the long-term aversive memory analyzed 24 h after training session. Results are expressed as mean ± SEM and are representative of at least two independent experiments (n = 7 per group). (GIF 26 kb)
Figure S5
Effect of MK801 in motor and exploratory activities following Plasmodium berghei ANKA (PbA) infection resolution by chloroquine (CQ) therapy. From 10 days after cessation of CQ treatment all mice were submitted to the open field task for general motor and exploratory activities analysis. No significant differences were found in a global activity, b stereotype movements, c locomotion, d mean velocity, or e in the number of rearing episodes. Results are expressed as mean ± SEM and are representative of at least two independent experiments (n = 8 per group). (GIF 73 kb)
Figure S6
MK801 effect in inflammatory response in the spleen of CM mice following Plasmodium berghei ANKA (PbA) infection resolution by chloroquine (CQ) therapy. Ten days after cessation of CQ therapy, all animals were culled and spleen was harvested; homogenized; and IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α levels were assessed by Cytometric Bead Array (CBA). Results are expressed as mean ± SEM and are representative of at least two independent experiments (n = 5 per group). Asterisk(s) indicate statistical differences where *p < 0.05, **p < 0.01, ***p < 0.001. (GIF 182 kb)
Figure S7
MK801 effect in inflammatory response in the serum of CM mice following Plasmodium berghei ANKA (PbA) infection resolution by chloroquine (CQ) therapy. Ten days after cessation of CQ therapy serum was collected and IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α levels were assessed by cytometric bead array (CBA). Results are expressed as mean ± SEM and are representative of at least two independent experiments (n = 5 per group). Asterisk(s) indicate statistical differences where *p < 0.05, **p < 0.01, ***p < 0.001. (GIF 73 kb)
Rights and permissions
About this article
Cite this article
de Miranda, A.S., Brant, F., Vieira, L.B. et al. A Neuroprotective Effect of the Glutamate Receptor Antagonist MK801 on Long-Term Cognitive and Behavioral Outcomes Secondary to Experimental Cerebral Malaria. Mol Neurobiol 54, 7063–7082 (2017). https://doi.org/10.1007/s12035-016-0226-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0226-3