Abstract
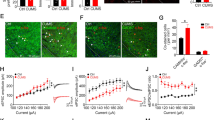
Src homolog domain-containing phosphatase 2 (Shp2) signals a variety of cellular and physiological functions including learning and memory. Dysregulation of ERK signaling is known to be responsible for the cognitive deficits associated with gain-of-function mutated Shp2 mimicking Noonan syndrome. However, here, we report that CaMKIIα-cre induced knockout (CaSKO) of Shp2 in hippocampal pyramidal neurons resulted in increased Src activity, upregulated phosphorylation of N-methyl-D-aspartate receptors (NMDARs) at Y1325 of GluN2A and at Y1472 of GluN2B, disrupted the balance of synaptic transmission, and impaired long-term potentiation and remote contextual fear memory. Administration of PP2, a specific Src family kinase inhibitor, reversed the tyrosine phosphorylation of NMDARs, restored basal synaptic transmission, and rescued the contextual fear memory deficit in CaSKO mice without altering the phospho-ERK level. Taken together, our results reveal a novel role of Shp2 in NMDAR-dependent synaptic function and fear memory via the Src signaling pathway rather than the ERK pathway, and suggest a complicated mechanism for Shp2-associated cognitive deficits.







Similar content being viewed by others
References
Voron DA, Hatfield HH, Kalkhoff RK (1976) Multiple lentigines syndrome. Case report and review of the literature. Am J Med 60(3):447–456. doi:10.1016/0002-9343(76)90764-6
Lee DA, Portnoy S, Hill P, Gillberg C, Patton MA (2005) Psychological profile of children with Noonan syndrome. Dev Med Child Neurol 47(1):35–38. doi:10.1111/j.1469-8749.2005.tb01037.x
Cesarini L, Alfieri P, Pantaleoni F, Vasta I, Cerutti M, Petrangeli V, Mariotti P, Leoni C et al (2009) Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade. Am J Med Genet A 149A(2):140–146. doi:10.1002/ajmg.a.32488
Pierpont EI, Pierpont ME, Mendelsohn NJ, Roberts AE, Tworog-Dube E, Seidenberg MS (2009) Genotype differences in cognitive functioning in Noonan syndrome. Genes Brain Behav 8(3):275–282. doi:10.1111/j.1601-183X.2008.00469.x
Alfieri P, Cesarini L, Mallardi M, Piccini G, Caciolo C, Leoni C, Mirante N, Pantaleoni F et al (2011) Long term memory profile of disorders associated with dysregulation of the RAS-MAPK signaling cascade. Behav Genet 41(3):423–429. doi:10.1007/s10519-011-9446-5
Sarkozy A, Digilio MC, Dallapiccola B (2008) Leopard syndrome. Orphanet J Rare Dis 3:13. doi:10.1186/1750-1172-3-13
Pagani MR, Oishi K, Gelb BD, Zhong Y (2009) The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell 139(1):186–198. doi:10.1016/j.cell.2009.08.033
Lee YS, Ehninger D, Zhou M, Oh JY, Kang M, Kwak C, Ryu HH, Butz D et al (2014) Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome. Nat Neurosci 17(12):1736–1743. doi:10.1038/nn.3863
Neel BG, Gu H, Pao L (2003) The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci 28(6):284–293. doi:10.1016/S0968-0004(03)00091-4
Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, Luo J, Thompson JA et al (2004) Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell 13(3):341–355. doi:10.1016/S1097-2765(04)00050-4
Marin TM, Clemente CF, Santos AM, Picardi PK, Pascoal VD, Lopes-Cendes I, Saad MJ, Franchini KG (2008) Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways. Circ Res 103(8):813–824. doi:10.1161/CIRCRESAHA.108.179754
Jo A, Park H, Lee SH, Ahn SH, Kim HJ, Park EM, Choi YH (2014) SHP-2 binds to caveolin-1 and regulates Src activity via competitive inhibition of CSK in response to H2O2 in astrocytes. PLoS One 9(3):e91582. doi:10.1371/journal.pone.0091582
Lu YM, Roder JC, Davidow J, Salter MW (1998) Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science 279(5355):1363–1367. doi:10.1126/science.279.5355.1363
Yamazaki Y, Jia Y, Wong JK, Sumikawa K (2006) Chronic nicotine-induced switch in Src-family kinase signaling for long-term potentiation induction in hippocampal CA1 pyramidal cells. Eur J Neurosci 24(11):3271–3284. doi:10.1111/j.1460-9568.2006.05213.x
Babus LW, Little EM, Keenoy KE, Minami SS, Chen E, Song JM, Caviness J, Koo SY et al (2011) Decreased dendritic spine density and abnormal spine morphology in Fyn knockout mice. Brain Res 1415:96–102. doi:10.1016/j.brainres.2011.07.059
Huang CC, Hsu KS (1999) Protein tyrosine kinase is required for the induction of long-term potentiation in the rat hippocampus. J Physiol 520(Pt 3):783–796. doi:10.1111/j.1469-7793.1999.00783.x
Sinai L, Duffy S, Roder JC (2010) Src inhibition reduces NR2B surface expression and synaptic plasticity in the amygdala. Learn Mem 17(8):364–371. doi:10.1101/lm.1765710
Yang K, Trepanier C, Sidhu B, Xie YF, Li H, Lei G, Salter MW, Orser BA et al (2012) Metaplasticity gated through differential regulation of GluN2A versus GluN2B receptors by Src family kinases. EMBO J 31(4):805–816. doi:10.1038/emboj.2011.453
Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3(7):661–669. doi:10.1038/76615
Lin SY, Wu K, Len GW, Xu JL, Levine ES, Suen PC, Mount HT, Black IB (1999) Brain-derived neurotrophic factor enhances association of protein tyrosine phosphatase PTP1D with the NMDA receptor subunit NR2B in the cortical postsynaptic density. Brain Res Mol Brain Res 70(1):18–25. doi:10.1016/S0169-328X(99)00122-9
Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB (2012) Spinal SIRPalpha1-SHP2 interaction regulates spinal nerve ligation-induced neuropathic pain via PSD-95-dependent NR2B activation in rats. Pain 153(5):1042–1053. doi:10.1016/j.pain.2012.02.006
Ding X, Cai J, Li S, Liu XD, Wan Y, Xing GG (2014) BDNF contributes to the development of neuropathic pain by induction of spinal long-term potentiation via SHP2 associated GluN2B-containing NMDA receptors activation in rats with spinal nerve ligation. Neurobiol Dis 73C:428–451. doi:10.1016/j.nbd.2014.10.025
Wang YT, Salter MW (1994) Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 369(6477):233–235. doi:10.1038/369233a0
Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T et al (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem 276(1):693–699. doi:10.1074/jbc.M008085200
Lu W, Fang W, Li J, Zhang B, Yang Q, Yan X, Peng L, Ai H et al (2015) Phosphorylation of tyrosine Y1070 at the GluN2B subunit is regulated by synaptic activity and critical for surface expression of NMDA receptors. J Biol Chem. doi:10.1074/jbc.M115.663450
Ai H, Lu W, Ye M, Yang W (2013) Synaptic non-GluN2B-containing NMDA receptors regulate tyrosine phosphorylation of GluN2B 1472 tyrosine site in rat brain slices. Neurosci Bull 29(5):614–620. doi:10.1007/s12264-013-1337-8
Taniguchi S, Nakazawa T, Tanimura A, Kiyama Y, Tezuka T, Watabe AM, Katayama N, Yokoyama K et al (2009) Involvement of NMDAR2A tyrosine phosphorylation in depression-related behaviour. EMBO J 28(23):3717–3729. doi:10.1038/emboj.2009.300
Yang M, Leonard JP (2001) Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-src. J Neurochem 77(2):580–588. doi:10.1016/S0959-4388(00)00216-6
Ali DW, Salter MW (2001) NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol 11(3):336–342. doi:10.1016/S0959-4388(00)00216-6
Trepanier CH, Jackson MF, MacDonald JF (2012) Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J 279(1):12–19. doi:10.1111/j.1742-4658.2011.08391.x
Baum ML, Kurup P, Xu J, Lombroso PJ (2010) A STEP forward in neural function and degeneration. Commun Integr Biol 3(5):419–422. doi:10.4161/cib.3.5.12692
Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ (2012) Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev 64(1):65–87. doi:10.1124/pr.110.003053
Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ (2005) The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 47(6):845–857. doi:10.1016/j.neuron.2005.08.016
Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM et al (2007) Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci 10(7):880–886. doi:10.1038/nn1914
Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD (2009) Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 158(4):1446–1459. doi:10.1016/j.neuroscience.2008.11.006
Goebel SM, Alvestad RM, Coultrap SJ, Browning MD (2005) Tyrosine phosphorylation of the N-methyl-D-aspartate receptor is enhanced in synaptic membrane fractions of the adult rat hippocampus. Brain Res Mol Brain Res 142(1):65–79. doi:10.1016/j.molbrainres.2005.09.012
Zhang EE, Chapeau E, Hagihara K, Feng GS (2004) Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A 101(45):16064–16069. doi:10.1073/pnas.0405041101
do Carmo JM, da Silva AA, Sessums PO, Ebaady SH, Pace BR, Rushing JS, Davis MT, Hall JE (2014) Role of Shp2 in forebrain neurons in regulating metabolic and cardiovascular functions and responses to leptin. Int J Obes 38(6):775–783. doi:10.1038/ijo.2013.177
Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS (2007) Labeling of dendritic spines with the carbocyanine dye DiI for confocal microscopic imaging in lightly fixed cortical slices. J Neurosci Methods 162(1–2):237–243. doi:10.1016/j.jneumeth.2007.01.016
Forlano PM, Woolley CS (2010) Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518(8):1330–1348. doi:10.1002/cne.22279
Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB (1997) The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 51(1):79–86. doi:10.1124/mol.51.1.79
Pan YW, Chan GC, Kuo CT, Storm DR, Xia Z (2012) Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen-activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J Neurosci 32(19):6444–6455. doi:10.1523/JNEUROSCI.6076-11.2012
Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K et al (2006) NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J 25(12):2867–2877. doi:10.1038/sj.emboj.7601156
Bourne J, Harris KM (2007) Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17(3):381–386. doi:10.1016/j.conb.2007.04.009
Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA (2011) Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron 71(6):1085–1101. doi:10.1016/j.neuron.2011.08.007
Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14(6):383–400. doi:10.1038/nrn3504
Hansen KB, Ogden KK, Yuan H, Traynelis SF (2014) Distinct functional and pharmacological properties of triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron 81(5):1084–1096. doi:10.1016/j.neuron.2014.01.035
Vergnano AM, Rebola N, Savtchenko LP, Pinheiro PS, Casado M, Kieffer BL, Rusakov DA, Mulle C et al (2014) Zinc dynamics and action at excitatory synapses. Neuron 82(5):1101–1114. doi:10.1016/j.neuron.2014.04.034
Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106(2):274–285. doi:10.1037//0735-7044.106.2.274
Barki-Harrington L, Elkobi A, Tzabary T, Rosenblum K (2009) Tyrosine phosphorylation of the 2B subunit of the NMDA receptor is necessary for taste memory formation. J Neurosci 29(29):9219–9226. doi:10.1523/JNEUROSCI.5667-08.2009
Lu W, Ai H, Peng L, Wang JJ, Zhang B, Liu X, Luo JH (2015) A novel phosphorylation site of N-methyl-d-aspartate receptor GluN2B at S1284 is regulated by Cdk5 in neuronal ischemia. Exp Neurol 271:251–258. doi:10.1016/j.expneurol.2015.06.016
Brandvold KR, Steffey ME, Fox CC, Soellner MB (2012) Development of a highly selective c-Src kinase inhibitor. ACS Chem Biol 7(8):1393–1398. doi:10.1021/cb300172e
Kusakari S, Saitow F, Ago Y, Shibasaki K, Sato-Hashimoto M, Matsuzaki Y, Kotani T, Murata Y et al (2015) Shp2 in forebrain neurons regulates synaptic plasticity, locomotion, and memory formation in mice. Mol Cell Biol 35(9):1557–1572. doi:10.1128/MCB.01339-14
Stewart RA, Sanda T, Widlund HR, Zhu S, Swanson KD, Hurley AD, Bentires-Alj M, Fisher DE et al (2010) Phosphatase-dependent and -independent functions of Shp2 in neural crest cells underlie LEOPARD syndrome pathogenesis. Dev Cell 18(5):750–762. doi:10.1016/j.devcel.2010.03.009
Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5(4):317–328. doi:10.1038/nrn1368
Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA (2008) Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci 28(2):415–424. doi:10.1523/JNEUROSCI.1900-07.2008
Xu F, Plummer MR, Len GW, Nakazawa T, Yamamoto T, Black IB, Wu K (2006) Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res 1121:22–34. doi:10.1016/j.brainres.2006.08.129
Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW et al (2005) Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8(8):1051–1058. doi:10.1038/nn1503
Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, Oksenberg D, Nikolich K (2006) Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP). Eur J Neurosci 23(11):2847–2856. doi:10.1111/j.1460-9568.2006.04837.x
Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ (2001) Molecular determinants of NMDA receptor internalization. Nat Neurosci 4(8):794–802. doi:10.1038/90498
Grosshans DR, Clayton DA, Coultrap SJ, Browning MD (2002) LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci 5(1):27–33. doi:10.1038/nn779
Yan YG, Zhang J, Xu SJ, Luo JH, Qiu S, Wang W (2014) Clustering of surface NMDA receptors is mainly mediated by the C-terminus of GluN2A in cultured rat hippocampal neurons. Neurosci Bull 30(4):655–666. doi:10.1007/s12264-014-1450-8
Dupuis JP, Ladepeche L, Seth H, Bard L, Varela J, Mikasova L, Bouchet D, Rogemond V et al (2014) Surface dynamics of GluN2B-NMDA receptors controls plasticity of maturing glutamate synapses. EMBO J. doi:10.1002/embj.201386356
McCormack SG, Stornetta RL, Zhu JJ (2006) Synaptic AMPA receptor exchange maintains bidirectional plasticity. Neuron 50(1):75–88. doi:10.1016/j.neuron.2006.02.027
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5(3):173–183. doi:10.1038/nrn1346
Stornetta RL, Zhu JJ (2011) Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist : A Rev J Bringing Neurobiol, Neurol Psychiatry 17(1):54–78. doi:10.1177/1073858410365562
Wang G, Bochorishvili G, Chen Y, Salvati KA, Zhang P, Dubel SJ, Perez-Reyes E, Snutch TP et al (2015) CaV3.2 calcium channels control NMDA receptor-mediated transmission: a new mechanism for absence epilepsy. Genes Dev 29(14):1535–1551. doi:10.1101/gad.260869.115
Sheng Y, Zhang L, Su SC, Tsai LH, Julius Zhu J (2015) Cdk5 is a new rapid synaptic homeostasis regulator capable of initiating the early Alzheimer-like pathology. Cereb Cortex. doi:10.1093/cercor/bhv032
Acknowledgments
We thank Gensheng Feng (University of California San Diego) for the Shp2 flox/flox mice and Zhiqi Xiong (Institute of Neuroscience, Chinese Academy of Sciences) for the CaMKII-Cre mice. We thank Dr. J Julius Zhu (University of Virginia) and Dr. I.C. Bruce for critical reading of this manuscript.
Author Contributions
W.Y., X.Y.Y., and J.H.L. conceived and designed the experiments and wrote the manuscript; X.Y.Y., W.Y., W.C., and S.L. performed behavioral experiments; X.Y.Y., Q.Y., and W.C. performed electrophysiology experiments; B.Z., W.L., and L.P. performed biochemical experiments; X.Y.Y. and W.Y.Y. performed immunocytochemical experiments; Y.H.K. provided Shp2 FloxP/FloxP mice; X.Y.Y., B.Z., and S.L. analyzed data; and X.Y.Y., S.L., W.Y.Y., and W.C. did the genotyping of mice. W.Y. and J.H.L. supervised the studies in Zhejiang University; S.T.L. supervised the studies in Shanghai Jiaotong University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by grants from the Natural Science Foundation of China (81671162, 91232303, and 81221003 to J.H.L. and 30900418 to W.Y.), the National Basic Research Program of China (grant numbers 2010CB912002 and 2014CB910300 to J.H.L., 2013CB910204 to W.Y., and 2010CB912004 to Y.H.K.), and Fundamental Research Funds for the Central Universities of China.
Conflict of Interest
The authors declare no competing financial interest.
Additional information
Xunyi Yan and Bin Zhang contributed equally to this work.
Electronic supplementary material
.
ESM 1
(DOC 4944 kb)
Rights and permissions
About this article
Cite this article
Yan, X., Zhang, B., Lu, W. et al. Increased Src Family Kinase Activity Disrupts Excitatory Synaptic Transmission and Impairs Remote Fear Memory in Forebrain Shp2-Deficient Mice. Mol Neurobiol 54, 7235–7250 (2017). https://doi.org/10.1007/s12035-016-0222-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0222-7