Abstract
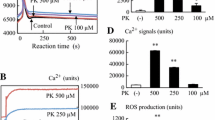
Phytanic acid, a saturated branched chain fatty acid and a major constituent of human diet, is predominantly found in dairy products, meat, and fish. It is a degradation product from the phytol side chain of chlorophyll. Degradation of PA is known to occur mainly in peroxisomes via α-oxidation and in mitochondria via β-oxidation. Due to its β-methyl group present at the 3-position of the carbon atoms, PA cannot be β-oxidized. Although alteration in the metabolism of PA may play an important role in neurodegeneration, the exact mechanism behind it remains to be evaluated. In this study, we have described the potential of PA to induce neurotoxicity as an in vitro model (neuronal cell line, SH-SY5Y cells). Cells were pretreated with melatonin (10 μM) for 1 h followed by with and without PA (100 μM) for 24 h. In the present study, our data has confirmed that PA markedly increased both intracellular reactive oxygen species and reactive nitrogen species levels. Our results have shown that PA treatment did not induce cell death by cleavage of caspase-3/PARP-1 mediated by mitochondria through intrinsic pathways; however, PA induced nitric oxide-dependent apoptosis in SH-SY5Y cells. Additionally, melatonin pretreatment reduced the cell death in SH-SY5Y cells. Melatonin also effectively exerted an antiapoptotic and anti-inflammatory action by regulating Bax, Bcl-2, p-NFκB, and iNOS expressions in SH-SY5Y cells. These results suggested that melatonin acted as an antioxidative and antiapoptotic agent by modulating ROS, apoptotic proteins, and inflammatory responses under BCFA-induced neurotoxic conditions. The protective effects of melatonin depend on direct scavenging activity of free radicals and indirect antioxidant effects. Further deciphering of the cellular and molecular mechanism associated with neuroprotection by melatonin is warranted in BCFA-induced neurotoxicity.





Similar content being viewed by others
References
Ran-Ressler RR, Devapatla S, Lawrence P, Brenna JT (2008) Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr Res 64:605–609
Ran-Ressler RR, Sim D, O’Donnell-Megaro AM, Bauman DE, Barbano DM, Brenna JT (2011) Branched chain fatty acid content of United States retail cow’s milk and implications for dietary intake. Lipids 46:569–576
Ferdinandusse S, Rusch H, Van Lint AEM, Dacremont G, Wanders RJA, Vreken P (2002) Stereochemistry of the peroxisomal branched-chain fatty acid alpha- and beta-oxidation systems in patients suffering from different peroxisomal disorders. J Lipid Res 43:438–444
Idel S, Ellinghaus P, Wolfrum C, Nofer JR, Gloerich J, Assmann G, Spener F, Seedorf U (2002) Branched chain fatty acids induce nitric oxide-dependent apoptosis in vascular smooth muscle cells. J Biol Chem 277:49319–49325
Kruska N, Reiser G (2011) Phytanic acid and pristanic acid, branched-chain fatty acids associated with Refsum disease and other inherited peroxisomal disorders, mediate intracellular Ca2+ signaling through activation of free fatty acid receptor GPR40. Neurobiol Dis 43:465–472
Singh VK, Hattangady DS, Giotis ES, Singh AK, Chamberlain NR, Stuart MK, Wilkinson BJ (2008) Insertional inactivation of branched-chain alpha-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl Environ Microbiol 74:5882–5890
Wongtangtintharn S, Oku H, Iwasaki H, Toda T (2004) Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J Nutr Sci Vitaminol 50:137–143
Hellgren LI (2010) Phytanic acid—an overlooked bioactive fatty acid in dairy fat? Ann N Y Acad Sci 1190:42–49
Yepuri NR, Holt SA, Moraes G, Holden PJ, Hossain KR, Valenzuela SM, James M, Darwish TA (2014) Stereoselective synthesis of perdeuterated phytanic acid, its phospholipid derivatives and their formation into lipid model membranes for neutron reflectivity studies. Chem Phys Lipids 183:22–33
Kataria Y, Wright M, Deaton RJ, Rueter EE, Rybicki BA, Moser AB, Ananthanrayanan V, Gann PH (2015) Dietary influences on tissue concentrations of phytanic acid and AMACR expression in the benign human prostate. Prostate 75:200–210
Borges CG, Canani CR, Fernandes CG, Zanatta Â, Seminotti B, Ribeiro CAJ, Leipnitz G, Vargas CR et al (2015) Reactive nitrogen species mediate oxidative stress and astrogliosis provoked by in vivo administration of phytanic acid in cerebellum of adolescent rats: a potential contributing pathomechanism of cerebellar injury in peroxisomal disorders. Neuroscience 304:122–132
Busanello ENB, Amaral AU, Tonin AM, Zanatta Â, Viegas CM, Vargas CR, Wajner M (2012) Disruption of mitochondrial homeostasis by phytanic acid in cerebellum of young rats. Cerebellum 12:362–369
Leipnitz G, Amaral AU, Zanatta A, Seminotti B, Fernandes CG, Knebel LA, Vargas CR, Wajner M (2010) Neurochemical evidence that phytanic acid induces oxidative damage and reduces the antioxidant defenses in cerebellum and cerebral cortex of rats. Life Sci 87:275–280
Rönicke S, Kruska N, Kahlert S, Reiser G (2009) The influence of the branched-chain fatty acids pristanic acid and Refsum disease-associated phytanic acid on mitochondrial functions and calcium regulation of hippocampal neurons, astrocytes, and oligodendrocytes. Neurobiol Dis 36:401–410
Kahlert S, Schonfeld P, Reiser G (2005) The Refsum disease marker phytanic acid, a branched chain fatty acid, affects Ca2+ homeostasis and mitochondria, and reduces cell viability in rat hippocampal astrocytes. Neurobiol Dis 18:110–118
Reiser G, Schönfeld P, Kahlert S (2006) Mechanism of toxicity of the branched-chain fatty acid phytanic acid, a marker of Refsum disease, in astrocytes involves mitochondrial impairment. Int J Dev Neurosci 24:113–122
Grings M, Tonin AM, Knebel LA, Zanatta A, Moura AP, Filho CSD, Wajner M, Leipnitz G (2012) Phytanic acid disturbs mitochondrial homeostasis in heart of young rats: a possible pathomechanism of cardiomyopathy in Refsum disease. Mol Cell Biochem 366:335–343
Schönfeld P, Reiser G (2006) Rotenone-like action of the branched-chain phytanic acid induces oxidative stress in mitochondria. J Biol Chem 281:7136–7142
Komen JC, Distelmaier F, Koopman WJH, Wanders RJA, Smeitink J, Willems PHMG (2007) Phytanic acid impairs mitochondrial respiration through protonophoric action. Cell Mol Life Sci 64:3271–3281
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53:S26–S36
Glade MJ, Smith K, Meguid MMA (2015) A glance at … nutritional antioxidants and testosterone secretion. Nutrition 31:1295–1298
Zhang HM, Zhang Y (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57:131–146
Kantar S, Türközkan N, Bircan FS, Paşaoğlu ÖT (2015) Beneficial effects of melatonin on serum nitric oxide, homocysteine, and ADMA levels in fructose-fed rats. Pharm Biol 53:1035–1041
Waseem M, Tabassum H, Parvez S (2016) Neuroprotective effects of melatonin as evidenced by abrogation of oxaliplatin induced behavioral alterations, mitochondrial dysfunction and neurotoxicity in rat brain. Mitochondrion 30:168–176
López A, García JA, Escames G, Venegas C, Ortiz F, López LC, Acuña-Castroviejo D (2009) Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res 46:188–198
Chen J, Wang L, Wu C, Hu Q, Gu C, Yan F, Li J, Yan W et al (2014) Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res 56:12–19
Karbownik M, Tan DX, Reiter RJ (2000) Melatonin reduces the oxidation of nuclear DNA and membrane lipids induced by the carcinogen delta-aminolevulinic acid. Int J Cancer 88:7–11
Pan X, Zhu L, Lu H, Wang D, Lu Q, Yan H (2015) Melatonin attenuates oxidative damage induced by acrylamide in vitro and in vivo. Oxidative Med Cell Longev 703709
Huang S, He H, Zou K, Bai C, Xue Y, Wang J, Chen J (2014) Protective effect of tomatine against hydrogen peroxide-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. J Pharm Pharmacol 66:844–854
Wisessmith W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B (2009) Melatonin reduces induction of Bax, caspase and cell death in methamphetamine-treated human neuroblastoma SH-SY5Y cultured cells. J Pineal Res 46:433–440
Schönfeld P, Kahlert S, Reiser G (2006) A study of the cytotoxicity of branched-chain phytanic acid with mitochondria and rat brain astrocytes. Exp Gerontol 41:688–696
Moravčík R, Okuliarová M, Kováčová E, Zeman M (2014) Diquat-induced cytotoxicity on Vero and HeLa cell lines: effect of melatonin and dihydromelatonin. Interdiscip Toxicol 7:184–188
Yew MY, Koh RY, Chye SM, Othman I, Ngi KY (2014) Edible bird's nest ameliorates oxidative stress-induced apoptosis in SH-SY5Y human neuroblastoma cells. BMC Complement Altern Med 13:391
Suematsu N, Hosoda M, Fujimori K (2011) Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci Lett 504:223–227
Venkataramana M, Chandra Nayaka S, Anand T, Rajesh R, Aiyaz M, Divakara ST, Murali HS, Prakash HS et al (2014) Zearalenone induced toxicity in SHSY-5Y cells: the role of oxidative stress evidenced by N-acetyl cysteine. Food Chem Toxicol 65:335–342
Czapski GA, Sun GY, Strosznajder JB (2002) Inhibition of N-methyl-D-aspartic acid-nitric oxide synthase in rat hippocampal slices by ethanol: evidence for the involvement of tetrahydrobiopterin but not lipid peroxidation. J Biomed Sci 9:3–9
Sahu U, Sidhar H, Ghate PS, Advirao GM, Raghavan SC, Giri RK (2013) A novel anticancer agent, 8-Methoxypyrimido[4′,5':4,5]thieno(2,3-b) Quinoline-4(3H)-one induces neuro 2a neuroblastoma cell death through p53-dependent, caspase dependent and -independent apoptotic pathways. PLoS One 8:e66430
Bradford M (1976) Rapid and sensitive method for quantification of microgram quantities of protein utilizing principle of protein-dye-binding. Anal Biochem 72:248–254
Schönfeld P, Kahlert S, Reiser G (2004) In brain mitochondria the branched-chain fatty acid phytanic acid impairs energy transduction and sensitizes for permeability transition. Biochem J 383:121–128
Nirmaladevi D, Venkataramana M, Chandranayaka S, Ramesha A, Jameel NM, Srinivas C (2014) Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell Mol Neurobiol 34:973–985
Abarikwu SO, Farombi EO (2015) Atrazine induces apoptosis of SH-SY5Y human neuroblastoma cells via the regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway. Pestic Biochem Physiol 118:90–98
Martins JB, Bastos ML, Carvalho F, Capela JP (2013) Differential effects of methyl-4-phenylpyridinium ion, rotenone, and paraquat on differentiated SH-SY5Y cells. J Toxicol 2013:1–10
Yi F, He X, Wang D (2013) Lycopene protects against MPP+-induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochem Res 38:1747–1757
Tosun M, Soysal Y, Mas NG, Karabekir HS (2015) Comparison of the effects of 13-cis retinoic acid and melatonin on the viabilities of SH-SY5Y neuroblastoma cell line. J Korean Neurosurg Soc 57:147–151
Deniz E, Colakoglu N, Sari A, Sonmez MF, Tugrul I, Oktar S, İlhan S, Sahna E (2006) Melatonin attenuates renal ischemia–reperfusion injury in nitric oxide synthase inhibited rats. Acta Histochem 108:303–309
Titze-de-Almeida SS, Lustosa CF, Horst CH, Bel E, Del R, Titze-de-Almeida R (2014) Interferon gamma potentiates the injury caused by MPP(+) on SH-SY5Y cells, which is attenuated by the nitric oxide synthases inhibition. Neurochem Res 39:2452–2464
Song J, Kang SM, Lee KM, Lee JE (2015) The protective effect of melatonin on neural stem cell against LPS-induced inflammation. Biomed Res Int 854359
Garcimartín A, Merino JJ, González M, Sánchez-Reus M, Sánchez-Muniz FJ, Bastida S, Benedí J (2014) Organic silicon protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide effects. BMC Complement Altern Med 14:384
Wang XJ, Xu JX (2005) Salvianic acid a protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Neurosci Res 51:129–138
Abarikwu SO (2014) Protective effect of quercetin on atrazine-induced oxidative stress in the liver, kidney, brain, and heart of adult Wistar rats. Toxicol Int 21:148–155
Abarikwu SO, Farombi EO, Pant AB (2011) Biflavanone-kolaviron protects human dopaminergic SH-SY5Y cells against atrazine induced toxic insult. Toxicol Vitr 25:848–858
Bavithra S, Selvakumar K, Krishnamoorthy G, Venkataraman P, Arunakaran J (2013) Melatonin attenuates polychlorinated biphenyls induced apoptosis in the neuronal cells of cerebral cortex and cerebellum of adult male rats-in vivo. Environ Toxicol Pharmacol 36:152–163
Kauppinen T, Mauppinen TM (2007) Multiple roles for poly(ADP-ribose)polymerase-1 in neurological disease. Neurochem Int 50:954–958
Abdelsalam RM, Safar MM (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J Neurochem 133:700–707
Kermanian F, Soleimani M, Pourheydar B, Samzadeh-Kermani A, Mohammadzadeh F, Mehdizadeh M (2014) Effects of adenosine A2a receptor agonist and antagonist on cerebellar nuclear factor-kB expression preceded by MDMA toxicity. Med J Islam Repub Iran 28:120
Permpoonputtana K, Govitrapong P (2012) The anti-inflammatory effect of melatonin on methamphetamine-induced proinflammatory mediators in human neuroblastoma dopamine SH-SY5Y cell lines. Neurotox Res 23:189–199
Song JX, Shaw PC, Sze CW, Tong Y, Yao XS, Ngi TB, Zhang YB (2010) Chrysotoxine, a novel bibenzyl compound, inhibits 6-hydroxydopamine induced apoptosis in SH-SY5Y cells via mitochondria protection and NF-κB modulation. Neurochem Int 57:676–689
Abbasi Habashi S, Sabouni F, Moghimi A, Ansari Majd S (2016) Modulation of lipopolysaccharide stimulated nuclear factor kappa B mediated iNOS/NO production by bromelain in rat primary microglial cells. Iran Biomed J 20:33–40
Acknowledgments
The authors would like to thank the funding agencies involved in supporting SP (SERB-EMR Grant 2016/001070/HS) and SC (ICMR-Senior Research Fellowship 45/47/2013-PHA/BMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Shaista Chaudhary and Upasana Sahu contributed equally to the study
Rights and permissions
About this article
Cite this article
Chaudhary, S., Sahu, U., Kar, S. et al. Phytanic Acid-Induced Neurotoxicological Manifestations and Apoptosis Ameliorated by Mitochondria-Mediated Actions of Melatonin. Mol Neurobiol 54, 6960–6969 (2017). https://doi.org/10.1007/s12035-016-0209-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0209-4