Abstract
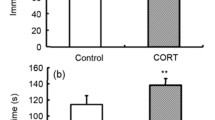
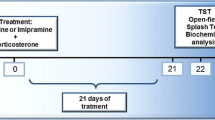
The benefits of creatine supplementation have been reported in a broad range of central nervous system diseases, including depression, although the mechanisms underlying these effects remain to be understood. In the present study, we investigated the ability of creatine to counteract the morphological and behavioral effects elicited by chronic administration of corticosterone (CORT, 20 mg/kg, p.o.) for 21 days to mice, a pharmacological model of depression that mimics exposure to stress. CORT treatment increased immobility time in the tail suspension test (TST) and forced swim test (FST), as well as latency to immobility in the FST, and decreased the sucrose consumption in the sucrose preference test (SPT). These behavioral effects were associated with decreased hippocampal cell proliferation and neuronal differentiation and increased glial fibrillary acid protein (GFAP) immunostaining (suggestive of astrogliosis) in dentate gyrus (DG) of the hippocampus. These CORT-induced alterations were abolished by treatment with either fluoxetine (a conventional antidepressant) or creatine for 21 days (both 10 mg/kg, p.o.). In addition, fluoxetine, but not creatine, was able to reverse the CORT-induced reduction in serum CORT levels. Collectively, our results suggest that creatine produces morphological alterations that contribute to the improvement of depressive-like behaviors triggered by chronic CORT administration in mice.




Similar content being viewed by others
Abbreviations
- Akt/PKB:
-
Protein kinase B
- ANOVA:
-
Analysis of variance
- BDNF:
-
Brain-derived neurotrophic factor
- CaMK-II:
-
Ca2+/calmodulin-dependent protein kinase II
- CORT:
-
Corticosterone
- DAB:
-
2,2-Diaminobenzidine
- DG:
-
Dentate gyrus
- DMSO:
-
Dimethyl sulfoxide
- FST:
-
Forced swimming test
- GFAP:
-
Glial fibrillary acid protein
- HPA:
-
Hypothalamic–pituitary–adrenal
- MEK1/2:
-
Mitogen-activated protein kinase kinase 1/2
- mTOR:
-
Mammalian target of rapamycin
- NeuroD:
-
Neurogenic differentiation protein
- NHS:
-
Normal horse serum
- OFT:
-
Open field test
- p.o.:
-
Per os (oral route)
- PBS:
-
Phosphate-buffered saline
- PBS-Tx:
-
Phosphate buffer saline-Triton X-100
- PI3K:
-
Phosphatidylinositol-3-kinase
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- S.E.M.:
-
Standard error of the mean
- SCT:
-
Sucrose consumption test
- SGZ:
-
Subgranular zone
- SSRIs:
-
Selective serotonin reuptake inhibitors
- SVZ:
-
Subventricular zone
- TBS:
-
Tris-buffered saline
- TST:
-
Tail suspension test
References
Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK (2003) The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry 64(12):1465–1475. doi:10.4088/JCP.14m09298
Viana MC, Gruber MJ, Shahly V, Alhamzawi A, Alonso J, Andrade LH, Angermeyer MC, Benjet C, Bruffaerts R, Caldas-de-Almeida JM, Girolamo G, Jonge P, Ferry F, Florescu S, Gureje O, Haro JM, Hinkov H, Hu C, Karam EG, Lepine JP, Levinson D, Posada-Villa J, Sampson NA, Kessler RC (2013) Family burden related to mental and physical disorders in the world: results from the WHO World Mental Health (WMH) surveys. Rev Bras Psiquiatr 35(2):115–125. doi:10.1590/1516-4446-2012-0919
Nakajima S, Suzuki T, Watanabe K, Kashima H, Uchida H (2010) Accelerating response to antidepressant treatment in depression: a review and clinical suggestions. Prog Neuro-Psychopharmacol Biol Psychiatry 34(2):259–264. doi:10.1016/j.pnpbp.2009.12.001
Covington HE 3rd, Vialou V, Nestler EJ (2010) From synapse to nucleus: novel targets for treating depression. Neuropharmacology 58(4–5):683–693. doi:10.1016/j.neuropharm.2009.12.004
Tanti A, Belzung C (2013) Neurogenesis along the septo-temporal axis of the hippocampus: are depression and the action of antidepressants region-specific? Neuroscience 252:234–252. doi:10.1016/j.neuroscience.2013.08.017
Lie DC, Song H, Colamarino SA, Ming GL, Gage FH (2004) Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol 44:399–421. doi:10.1146/annurev.pharmtox.44.101802.121631
Kempermann G, Wiskott L, Gage FH (2004) Functional significance of adult neurogenesis. Curr Opin Neurobiol 14(2):186–191. doi:10.1016/j.conb.2004.03.001
Eisch AJ, Petrik D (2012) Depression and hippocampal neurogenesis: a road to remission? Science 338(6103):72–75. doi:10.1126/science.1222941
Cunha MP, Martin-de-Saavedra MD, Romero A, Egea J, Ludka FK, Tasca CI, Farina M, Rodrigues AL, Lopez MG (2014) Both creatine and its product phosphocreatine reduce oxidative stress and afford neuroprotection in an in vitro Parkinson's model. ASN neuro 6 (6). doi:10.1177/1759091414554945
Cunha MP, Pazini FL, Ludka FK, Rosa JM, Oliveira A, Budni J, Ramos-Hryb AB, Lieberknecht V, Bettio LE, Martin-de-Saavedra MD, Lopez MG, Tasca CI, Rodrigues AL (2015) The modulation of NMDA receptors and L-arginine/nitric oxide pathway is implicated in the anti-immobility effect of creatine in the tail suspension test. Amino Acids 47(4):795–811. doi:10.1007/s00726-014-1910-0
Cunha MP, Machado DG, Capra JC, Jacinto J, Bettio LEB, Rodrigues ALS (2012) Antidepressant-like effect of creatine in mice involves dopaminergic activation. J Psychopharmacol 26(11):1489–1501. doi:10.1177/0269881112447989
Pazini FL, Cunha MP, Rosa JM, Colla AR, Lieberknecht V, Oliveira A, Rodrigues AL (2015) Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via PI3K/Akt/mTOR pathway. Mol Neurobiol. doi:10.1007/s12035-015-9580-9
Kondo DG, Sung YH, Hellem TL, Fiedler KK, Shi X, Jeong EK, Renshaw PF (2011) Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord 135(1–3):354–361. doi:10.1016/j.jad.2011.07.010
Lyoo IK, Yoon S, Kim TS, Hwang J, Kim JE, Won W, Bae S, Renshaw PF (2012) A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am J Psychiatry 169(9):937–945. doi:10.1176/appi.ajp.2012.12010009
Sterner EY, Kalynchuk LE (2010) Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuro-Psychopharmacol Biol Psychiatry 34(5):777–790. doi:10.1016/j.pnpbp.2010.03.005
Mao QQ, Huang Z, Zhong XM, Xian YF, Ip SP (2014) Piperine reverses the effects of corticosterone on behavior and hippocampal BDNF expression in mice. Neurochem Int 74:36–41. doi:10.1016/j.neuint.2014.04.017
Zhao Y, Xie W, Dai J, Wang Z, Huang Y (2009) The varying effects of short-term and long-term corticosterone injections on depression-like behavior in mice. Brain Res 1261:82–90. doi:10.1016/j.brainres.2008.12.083
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581(1–2):113–120. doi:10.1016/j.ejphar.2007.12.005
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229(2):327–336. doi:10.1016/0014-2999(78)90414-4
Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues AL (2012) Depressive-like behavior induced by tumor necrosis factor-alpha in mice. Neuropharmacology 62(1):419–426. doi:10.1016/j.neuropharm.2011.08.018
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370
Rodrigues AL, Rocha JB, Mello CF, Souza DO (1996) Effect of perinatal lead exposure on rat behaviour in open-field and two-way avoidance tasks. Pharmacol Toxicol 79(3):150–156. doi:10.1111/j.1600-0773.1996.tb00259.x
Takatsu-Coleman AL, Patti CL, Zanin KA, Zager A, Carvalho RC, Borcoi AR, Ceccon LM, Berro LF, Tufik S, Andersen ML, Frussa-Filho R (2013) Short-term social isolation induces depressive-like behaviour and reinstates the retrieval of an aversive task: mood-congruent memory in male mice? J Psychiatr Neurosci : JPN 38(4):259–268. doi:10.1503/jpn.120050
Weiss JM (1997) Does decreased sucrose intake indicate loss of preference in CMS model? Psychopharmacology 134(4):368–370. doi:10.1007/s002130050472
Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002) The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Meth 115(1):97–105. doi:10.1016/S0165-0270(02)00007-9
Miyata T, Maeda T, Lee JE (1999) NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Gene Dev 13(13):1647–1652. doi:10.1101/gad.13.13.1647
Paxinos G, Franklin KBJ, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Gil-Mohapel J, Brocardo PS, Choquette W, Gothard R, Simpson JM, Christie BR (2013) Hippocampal neurogenesis levels predict WATERMAZE search strategies in the aging brain. PLoS One 8(9):e75125. doi:10.1371/journal.pone.0075125
de Oliveira J, Moreira EL, dos Santos DB, Piermartiri TC, Dutra RC, Pinton S, Tasca CI, Farina M, Prediger RD, de Bem AF (2014) Increased susceptibility to amyloid-beta-induced neurotoxicity in mice lacking the low-density lipoprotein receptor. J Alzheimers Dis: JAD 41(1):43–60. doi:10.3233/JAD-132228
Cryan JF, Page ME, Lucki I (2005) Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology 182(3):335–344. doi:10.1007/s00213-005-0093-5
Kelly KA, Miller DB, Bowyer JF, O'Callaghan JP (2012) Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. J Neurochem 122(5):995–1009. doi:10.1111/j.1471-4159.2012.07864.x
Chatterjee S, Sikdar SK (2013) Corticosterone treatment results in enhanced release of peptidergic vesicles in astrocytes via cytoskeletal rearrangements. Glia 61(12):2050–2062. doi:10.1002/glia.22576
Hill AS, Sahay A, Hen R (2015) Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology ACNP 40(10):2368–2378. doi:10.1038/npp.2015.85
Jacobson L, Sapolsky R (1991) The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12(2):118–134. doi:10.1210/edrv-12-2-118
Brummelte S, Galea LA (2010) Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 168(3):680–690. doi:10.1016/j.neuroscience.2010.04.023
Gilhooley MJ, Pinnock SB, Herbert J (2011) Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neurosci Lett 489(3):177–181. doi:10.1016/j.neulet.2010.12.011
Hanson ND, Owens MJ, Nemeroff CB (2011) Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology 36(13):2589–2602. doi:10.1038/npp.2011.220
Freitas AE, Egea J, Buendia I, Gomez-Rangel V, Parada E, Navarro E, Casas AI, Wojnicz A, Ortiz JA, Cuadrado A, Ruiz-Nuno A, Rodrigues AL, Lopez MG (2016) Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol Neurobiol 53(5):3030–3045. doi:10.1007/s12035-015-9182-6
Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR (2008) Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry 64(10):884–890. doi:10.1016/j.biopsych.2008.06.016
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62(4):479–493. doi:10.1016/j.neuron.2009.04.017
Gourley SL, Taylor JR (2009) Recapitulation and reversal of a persistent depression-like syndrome in rodents. Current protocols in neuroscience/editorial board, Jacqueline N Crawley [et al] Chapter 9: Unit 9 32. doi:10.1002/0471142301.ns093 2 s49
Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C (2008) Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64(4):293–301. doi:10.1016/j.biopsych.2008.02.022
Morgan VA, Mitchell PB, Jablensky AV (2005) The epidemiology of bipolar disorder: sociodemographic, disability and service utilization data from the Australian National Study of Low Prevalence (Psychotic) Disorders. Bipolar Disord 7(4):326–337. doi:10.1111/j.1399-5618.2005.00229.x
Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL (2007) Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci 27(31):8278–8285. doi:10.1523/JNEUROSCI.2121-07.2007
Bjornebekk A, Mathe AA, Brene S (2005) The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol 8(3):357–368. doi:10.1017/S1461145705005122
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20(24):9104–9110
Bettio LE, Cunha MP, Budni J, Pazini FL, Oliveira A, Colla AR, Rodrigues AL (2012) Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res 234(2):137–148. doi:10.1016/j.bbr.2012.06.021
Antonijevic IA (2006) Depressive disorders - is it time to endorse different pathophysiologies? Psychoneuroendocrinology 31(1):1–15. doi:10.1016/j.psyneuen.2005.04.004
Heuser IJE, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, Yassouridis A, Holsboer F (1996) Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am J Psychiatry 153(1):93–99. doi:10.1176/ajp.153.1.93
Holsboertrachsler E, Stohler R, Hatzinger M (1991) Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatry Res 38(2):163–171. doi:10.1016/0165-1781(91)90041-M
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476(7361):458–U112. doi:10.1038/nature10287
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C (2011) Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16(12):1177–1188. doi:10.1038/mp.2011.48
Sahay A, Hen R (2007) Adult hippocampal neurogenesis in depression. Nature Neurosci 10(9):1110–1115. doi:10.1038/nn1969
Diniz L, dos Santos TB, Britto LR, Cespedes IC, Garcia MC, Spadari-Bratfisch RC, Medalha CC, de Castro GM, Montesano FT, Viana MB (2013) Effects of chronic treatment with corticosterone and imipramine on fos immunoreactivity and adult hippocampal neurogenesis. Behav Brain Res 238:170–177. doi:10.1016/j.bbr.2012.10.024
Rainer Q, Xia L, Guilloux JP, Gabriel C, Mocaer E, Hen R, Enhamre E, Gardier AM, David DJ (2012) Beneficial behavioural and neurogenic effects of agomelatine in a model of depression/anxiety. Int J Neuropsychopharmacol 15(3):321–335. doi:10.1017/S1461145711000356
Robertson DA, Beattie JE, Reid IC, Balfour DJ (2005) Regulation of corticosteroid receptors in the rat brain: the role of serotonin and stress. Eur J Neurosci 21(6):1511–1520. doi:10.1111/j.1460-9568.2005.03990.x
Lin YL, Lin SY, Wang S (2012) Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain Behav Immun 26(3):459–468. doi:10.1016/j.bbi.2011.12.003
Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ (2004) Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull 64(4):303–308. doi:10.1016/j.brainresbull.2004.08.006
Paizanis E, Renoir T, Lelievre V, Saurini F, Melfort M, Gabriel C, Barden N, Mocaer E, Hamon M, Lanfumey L (2010) Behavioural and neuroplastic effects of the new-generation antidepressant agomelatine compared to fluoxetine in glucocorticoid receptor-impaired mice. Int J Neuropsychopharmacol 13(6):759–774. doi:10.1017/S1461145709990514
Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M (2013) Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci 33(7):2961–2972. doi:10.1523/JNEUROSCI.3878-12.2013
Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S (2012) Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology 37(10):2210–2221. doi:10.1038/npp.2012.70
Tanti A, Rainer Q, Minier F, Surget A, Belzung C (2012) Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology 63(3):374–384. doi:10.1016/j.neuropharm.2012.04.022
Memberg SP, Hall AK (1995) Proliferation, differentiation, and survival of rat sensory neuron precursors in vitro require specific trophic factors. Mol Cel Neurosci 6(4):323–335. doi:10.1006/mcne.1995.1025
Palmer TD, Takahashi J, Gage FH (1997) The adult rat hippocampus contains primordial neural stem cells. Mol Cel Neurosci 8(6):389–404. doi:10.1006/mcne.1996.0595
Takahashi J, Palmer TD, Gage FH (1999) Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol 38(1):65–81. doi:10.1002/(SICI)1097
Mao QQ, Xian YF, Ip SP, Tsai SH, Che CT (2010) Long-term treatment with peony glycosides reverses chronic unpredictable mild stress-induced depressive-like behavior via increasing expression of neurotrophins in rat brain. Behav Brain Res 210(2):171–177. doi:10.1016/j.bbr.2010.02.026
Cunha MP, Budni J, Pazini FL, Oliveira A, Rosa JM, Lopes MW, Leal RB, Rodrigues AL (2014) Involvement of PKA, PKC, CAMK-II and MEK1/2 in the acute antidepressant-like effect of creatine in mice. Pharmacol Rep : PR 66(4):653–659. doi:10.1016/j.pharep.2014.03.004
Cunha MP, Budni J, Ludka FK, Pazini FL, Rosa JM, Oliveira A, Lopes MW, Tasca CI, Leal RB, Rodrigues AL (2016) Involvement of PI3K/Akt signaling pathway and its downstream intracellular targets in the antidepressant-like effect of creatine. Mol Neurobiol 53(5):2954–2968. doi:10.1007/s12035-015-9192-4
Robel S, Sontheimer H (2015) Glia as drivers of abnormal neuronal activity. Nature neurosci 19(1):28–33. doi:10.1038/nn.4184
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35. doi:10.1007/s00401-009-0619-8
Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R (2014) Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19(6):699–709. doi:10.1038/mp.2013.155
Laping NJ, Teter B, Nichols NR, Rozovsky I, Finch CE (1994) Glial fibrillary acidic protein: regulation by hormones, cytokines, and growth factors. Brain Pathol 4(3):259–275. doi:10.1111/j.1750-3639.1994.tb00841.x
Leite MC, Brolese G, de Almeida LMV, Pinero CC, Gottfried C, Goncalves CA (2006) Ammonia-induced alteration in S100B secretion in astrocytes is not reverted by creatine addition. Brain Res Bull 70(2):179–185. doi:10.1016/j.brainresbull.2006.05.003
Lensman M, Korzhevskii DE, Mourovets VO, Kostkin VB, Izvarina N, Perasso L, Gandolfo C, Otellin VA, Polenov SA, Balestrino M (2006) Intracerebroventricular administration of creatine protects against damage by global cerebral ischemia in rat. Brain Res 1114:187–194. doi:10.1016/j.brainres.2006.06.103
Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14(11):1225–1236. doi:10.2174/13894501113149990156
Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E (2014) Antidepressants reduce neuroinflammatory responses and astroglial alpha-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia 62(2):317–337. doi:10.1002/glia.22610
Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31(8):1616–1626. doi:10.1038/sj.npp.1300982
Araya-Callis C, Hiemke C, Abumaria N, Flugge G (2012) Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology 224(1):209–222. doi:10.1007/s00213-012-2741-x
Acknowledgments
J.G.M. and A.L.S.R. acknowledge funding from the Science Without Borders funding program [Programa Ciência Sem Fronteiras/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Project no. 403120/2012-8] of the Brazilian Federal Government. This study was also supported by grants from CNPq (no. 308723/2013-9, no. 449436/2014-4) and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), NENASC Project (PRONEX-FAPESC/CNPq) no. 1262/2012-9. A.L.S.R. is CNPq Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holding that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Pazini, F.L., Cunha, M.P., Azevedo, D. et al. Creatine Prevents Corticosterone-Induced Reduction in Hippocampal Proliferation and Differentiation: Possible Implication for Its Antidepressant Effect. Mol Neurobiol 54, 6245–6260 (2017). https://doi.org/10.1007/s12035-016-0148-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0148-0