Abstract
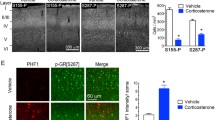
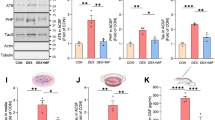
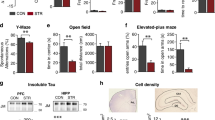
The exposure to high glucocorticoids (GC) triggers neuronal atrophy and cognitive deficits, but the exact cellular mechanisms underlying the GC-associated dendritic remodeling and spine loss are still poorly understood. Previous studies have implicated sustained GC elevations in neurodegenerative mechanisms through GC-evoked hyperphosphorylation of the cytoskeletal protein Tau while Tau mislocation has recently been proposed as relevant in Alzheimer’s disease (AD) pathology. In light of the dual cytoplasmic and synaptic role of Tau, this study monitored the impact of prolonged GC treatment on Tau intracellular localization and its phosphorylation status in different cellular compartments. We demonstrate, both by biochemical and ultrastructural analysis, that GC administration led to cytosolic and dendritic Tau accumulation in rat hippocampus, and triggered Tau hyperphosphorylation in epitopes related to its malfunction (Ser396/404) and cytoskeletal pathology (e.g., Thr231 and Ser262). In addition, we show, for the first time, that chronic GC administration also increased Tau levels in synaptic compartment; however, at the synapse, there was an increase in phosphorylation of Ser396/404, but a decrease of Thr231. These GC-triggered Tau changes were paralleled by reduced levels of synaptic scaffolding proteins such as PSD-95 and Shank proteins as well as reduced dendritic branching and spine loss. These in vivo findings add to our limited knowledge about the underlying mechanisms of GC-evoked synaptic atrophy and neuronal disconnection implicating Tau missorting in mechanism(s) of synaptic damage, beyond AD pathology.



Similar content being viewed by others
Abbreviations
- GC:
-
Glucocorticoids
- MT:
-
Microtubules
- AD:
-
Alzheimer’s disease
- MWM:
-
Morris water maze
- PSD-95:
-
Postsynaptic density protein 95
- DEX:
-
Dexamethasone
- GluN2B:
-
NMDA receptor 2B
- GluA2:
-
AMPA receptor 2
- ANOVA:
-
Analysis of variance
- TEM:
-
Transmission electron microscopy
References
Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N (2005) Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 25(34):7792–7800. doi:10.1523/JNEUROSCI.1598-05.2005
Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques P, Marques F, Palha JA, Cerqueira JJ et al (2013) Stress impact on resting state brain networks. PLoS ONE 8(6):e66500. doi:10.1371/journal.pone.0066500
Sousa N, Almeida OF (2012) Disconnection and reconnection: the morphological basis of (mal)adaptation to stress. Trends Neurosci 35(12):742–751. doi:10.1016/j.tins.2012.08.006
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–475. doi:10.1038/nrn1683
Gotz J, Xia D, Leinenga G, Chew YL, Nicholas H (2013) What Renders TAU Toxic. Front Neurol 4:72. doi:10.3389/fneur.2013.00072
Frandemiche ML, De Seranno S, Rush T, Borel E, Elie A, Arnal I, Lante F, Buisson A (2014) Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-beta oligomers. J Neurosci 34(17):6084–6097. doi:10.1523/JNEUROSCI.4261-13.2014
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC et al (2010) Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142(3):387–397. doi:10.1016/j.cell.2010.06.036
Kimura T, Whitcomb DJ, Jo J, Regan P, Piers T, Heo S, Brown C, Hashikawa T et al (2014) Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci 369(1633):20130144. doi:10.1098/rstb.2013.0144
Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA et al (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68(6):1067–1081. doi:10.1016/j.neuron.2010.11.030
Kimura T, Yamashita S, Fukuda T, Park JM, Murayama M, Mizoroki T, Yoshiike Y, Sahara N et al (2007) Hyperphosphorylated tau in parahippocampal cortex impairs place learning in aged mice expressing wild-type human tau. EMBO J 26(24):5143–5152. doi:10.1038/sj.emboj.7601917
Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT (2012) The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol 181(4):1426–1435. doi:10.1016/j.ajpath.2012.06.033
Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM (2013) Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J 32(22):2920–2937. doi:10.1038/emboj.2013.207
Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, Sousa N, Almeida OF (2011) Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J Neurosci 31(21):7840–7847. doi:10.1523/JNEUROSCI.0730-11.2011
Sotiropoulos I, Catania C, Riedemann T, Fry JP, Breen KC, Michaelidis TM, Almeida OF (2008) Glucocorticoids trigger Alzheimer disease-like pathobiochemistry in rat neuronal cells expressing human tau. J Neurochem 107(2):385–397. doi:10.1111/j.1471-4159.2008.05613.x
Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N (2007) Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex 17(9):1998–2006. doi:10.1093/cercor/bhl108
Sahara N, Murayama M, Higuchi M, Suhara T, Takashima A (2014) Biochemical distribution of tau protein in synaptosomal fraction of transgenic mice expressing human P301L tau. Front Neurol 5:26. doi:10.3389/fneur.2014.00026
Merino-Serrais P, Benavides-Piccione R, Blazquez-Llorca L, Kastanauskaite A, Rabano A, Avila J, DeFelipe J (2013) The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer’s disease. Brain 136(Pt 6):1913–1928. doi:10.1093/brain/awt088
Pooler AM, Usardi A, Evans CJ, Philpott KL, Noble W, Hanger DP (2012) Dynamic association of tau with neuronal membranes is regulated by phosphorylation. Neurobiol Aging 33(2):431. doi:10.1016/j.neurobiolaging.2011.01.005, e427-438
Mondragon-Rodriguez S, Trillaud-Doppia E, Dudilot A, Bourgeois C, Lauzon M, Leclerc N, Boehm J (2012) Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. J Biol Chem 287(38):32040–32053. doi:10.1074/jbc.M112.401240
Campbell SN, Zhang C, Monte L, Roe AD, Rice KC, Tache Y, Masliah E, Rissman RA (2015) Increased tau phosphorylation and aggregation in the hippocampus of mice overexpressing corticotropin-releasing factor. J Alzheimers Dis 43(3):967–976. doi:10.3233/JAD-141281
Morris M, Maeda S, Vossel K, Mucke L (2011) The many faces of tau. Neuron 70(3):410–426. doi:10.1016/j.neuron.2011.04.009
Hall GF, Chu B, Lee G, Yao J (2000) Human tau filaments induce microtubule and synapse loss in an in vivo model of neurofibrillary degenerative disease. J Cell Sci 113(Pt 8):1373–1387
Hall GF, Lee VM, Lee G, Yao J (2001) Staging of neurofibrillary degeneration caused by human tau overexpression in a unique cellular model of human tauopathy. Am J Pathol 158(1):235–246, doi:10.1016/S0002-9440(10)63962-4
Zempel H, Thies E, Mandelkow E, Mandelkow EM (2010) Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 30(36):11938–11950. doi:10.1523/JNEUROSCI.2357-10.2010
Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF (2009) The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry 14(1):95–105. doi:10.1038/sj.mp.4002101
Sotiropoulos I, Silva J, Kimura T, Rodrigues AJ, Costa P, Almeida OF, Sousa N, Takashima A (2015) Female hippocampus vulnerability to environmental stress, a precipitating factor in tau aggregation pathology. J Alzheimers Dis 43(3):763–774. doi:10.3233/JAD-140693
Luna-Munoz J, Chavez-Macias L, Garcia-Sierra F, Mena R (2007) Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J Alzheimer’s Dis 12(4):365–375
Lauckner J, Frey P, Geula C (2003) Comparative distribution of tau phosphorylated at Ser262 in pre-tangles and tangles. Neurobiol Aging 24(6):767–776
Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K (1998) Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys 357(2):299–309. doi:10.1006/abbi.1998.0813
Cho JH, Johnson GV (2004) Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J Neurochem 88(2):349–358
Yoshiyama Y, Zhang B, Bruce J, Trojanowski JQ, Lee VM (2003) Reduction of detyrosinated microtubules and Golgi fragmentation are linked to tau-induced degeneration in astrocytes. J Neurosci 23(33):10662–10671
Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, Almeida OF (2008) Stress and glucocorticoid footprints in the brain-the path from depression to Alzheimer’s disease. Neurosci Biobehav Rev 32(6):1161–1173. doi:10.1016/j.neubiorev.2008.05.007
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ et al (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316(5825):750–754. doi:10.1126/science.1141736
Miller EC, Teravskis PJ, Dummer BW, Zhao X, Huganir RL, Liao D (2014) Tau phosphorylation and tau mislocalization mediate soluble Abeta oligomer-induced AMPA glutamate receptor signaling deficits. Eur J Neurosci 39(7):1214–1224. doi:10.1111/ejn.12507
Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM (2006) Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci 26(35):9047–9056. doi:10.1523/JNEUROSCI.2797-06.2006
Takashima A, Noguchi K, Sato K, Hoshino T, Imahori K (1993) Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc Natl Acad Sci U S A 90(16):7789–7793
McKinney RA (2010) Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. J Physiol 588(Pt 1):107–116. doi:10.1113/jphysiol.2009.178905
Acknowledgments
We would like to thank Rui Fernandes for TEM technical support. IS was supported by the Portuguese Foundation for Science and Technology (FCT). This work was funded by the Portuguese Foundation for Science and Technology (FCT) (grant NMC-113934 to IS and grant SFRH/BPD/80118/2011 to JC), Canon Foundation and project DoIT - Desenvolvimento e Operacionalização da Investigação de Translação (N° do projeto 13853), funded by Fundo Europeu de Desenvolvimento Regional (FEDER) through the Programa Operacional Fatores de Competitividade (POFC).
Conflict of Interest
None of the authors report competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sara Pinheiro and Joana Silva contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pinheiro, S., Silva, J., Mota, C. et al. Tau Mislocation in Glucocorticoid-Triggered Hippocampal Pathology. Mol Neurobiol 53, 4745–4753 (2016). https://doi.org/10.1007/s12035-015-9356-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9356-2