Abstract
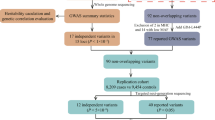
Genome-wide association studies (GWAS) on sporadic Parkinson’s disease (sPD) are mainly conducted in European and American populations at present, and the Han populations of Chinese mainland (HPCM) almost have not been studied yet. Here, we conducted a pooling GWAS combining a pathway analysis with 862,198 autosomal single nucleotide polymorphisms of IlluminaHumanOmniZhongHua-8 in 250 sPD and 250 controls from HPCM precluded toxicant exposure, age, and heavy coffee drinking habit interference. We revealed that among the 22 potential loci implicated, PRDM2/KIAA1026 (kgp8090149), TSG1/MANEA (kgp154172), PDE10A (kgp8130520), MDGA2 (rs9323124), ATPBD4/LOC100288892 (kgp11333367), ZFP64/TSHZ2 (kgp4156164), PAQR3/ARD1B (kgp9482779), FLJ23172/FNDC3B (kgp760898), C18orf1 (kgp348599), FLJ43860/NCRNA00051 (kgp4105983), CYP1B1/C2orf58 (kgp11353523), WNT9A/LOC728728 (rs849898), ANXA1/LOC100130911 (rs10746953), FLJ35379/LOC100132423 (kgp9550589), PLEKHN1 (kgp7172368), DMRT2/SMARCA2 (kgp10769919), ZNF396/INO80C (rs1362858), C3orf67/LOC339902 (rs6783485), LOC285194/IGSF11 (rs1879553), FGF10/MRPS30 (rs13153459), BARX1/PTPDC1 (kgp6542803), and COL5 A2 (rs11186), the peak significance was at the kgp4105983 of FLJ43860 gene in chromosome 8, the first top strongest associated locus with sPD was PRDM2 (kgp8090149) in chromosome 1, and the 24 pathways including 100 significantly associated genes were strongly associated with sPD from HPCM. The 40 genes were shared by at least two pathways. The most possible associated pathways with sPD were axon guidance, ECM-receptor interaction, neuroactive ligand-receptor interaction, tight junction, focal adhesion, gap junction, long-term depression, drug metabolism-cytochrome P450, adherens junction, endocytosis, and protein digestion and absorption. Our results indicated that these loci, pathways, and their related genes might be involved in the pathogenesis of sPD from HPCM and provided some novel evidences for further searching the genetic pathogenesis of sPD.









Similar content being viewed by others
References
Ali SF, Binienda ZK, Imam SZ (2011) Molecular aspects of dopaminergic neurodegeneration: gene-environment interaction in parkin dysfunction. Int J Environ Res Public Health 8:4702–4713. doi:10.3390/ijerph8124702
Liu G, Aliaga L, Cai H (2012) α-Synuclein, LRRK2 and their interplay in Parkinson’s disease. Future Neurol 7:145–153
Rochet JC, Hay BA, Guo M (2012) Molecular insights into Parkinson’s disease. Prog Mol Biol Transl Sci 107:125–188. doi:10.1016/B978-0-12-385883-2.00011-4
Saiki S, Sato S, Hattori N (2012) Molecular pathogenesis of Parkinson’s disease: update. J Neurol Neurosurg Psychiatry 83:430–436. doi:10.1136/jnnp-2011-301205
Saad M, Lesage S, Saint-Pierre A et al (2011) Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson’s disease in the European population. Hum Mol Genet 20:615–627. doi:10.1093/hmg/ddq497
Satake W, Nakabayashi Y, Mizuta I et al (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 41:1303–1307. doi:10.1038/ng.485
Simón-Sánchez J, Schulte C, Bras JM et al (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41:1308–1312. doi:10.1038/ng. 487
Simón-Sánchez J, van Hilten JJ, van de Warrenburg B et al (2011) Genome-wide association study confirms extant PD risk loci among the Dutch. Eur J Hum Genet 19:655–661. doi:10.1038/ejhg.2010.254
UK Parkinson’s Disease Consortium; Wellcome Trust Case Control Consortium 2, Spencer CC (2011) Dissection of the genetics of Parkinson’s disease identifies an additional association 5′ of SNCA and multiple associated haplotypes at 17q21. Hum Mol Genet 20:345–353. doi:10.1093/hmg/ddq469
Pankratz N, Wilk JB, Latourelle JC et al (2009) Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet 124:593–605. doi:10.1007/s00439-008-0582-9
Hamza TH, Zabetian CP, Tenesa A et al (2010) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42:781–785. doi:10.1038/ng.642
Edwards TL, Scott WK, Almonte C et al (2010) Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet 74:97–109. doi:10.1111/j.1469-1809.2009.00560.x
International Parkinson’s Disease Genomics Consortium (IPDGC); Wellcome Trust Case Control Consortium 2 (WTCCC2) (2011) A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet 7:e1002142. doi:10.1371/journal.pgen.1002142
International Parkinson Disease Genomics Consortium, Nalls MA, Plagnol V (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377:641–649. doi:10.1016/ S0140-6736(10)62345-8
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Szelinger S, Pearson JV, Craig DW (2011) Microarray-based genome-wide association studies using pooled DNA. Methods Mol Biol 700:49–60. doi:10.1007/978-1-61737-954- 3_4
Davis OS, Plomin R, Schalkwyk LC (2009) The SNPMaP package for R: a framework for genome-wide association using DNA pooling on microarrays. Bioinformatics 25:281–283. doi:10.1093/bioinformatics/btn587
Steer S, Abkevich V, Gutin A et al (2007) Genomic DNA pooling for whole-genome association scans in complex disease: empirical demonstration of efficacy in rheumatoid arthritis. Genes Immun 8:57–68
Wacholder S, Chanock S, Garcia-Closas M et al (2004) Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst 96:434–442
Wang K, Li M, Bucan M (2007) Pathway-based approaches for analysis of genomewide association studies. Am J Hum Genet 81:1278–1283
Schipper HM, Song W, Zukor H et al (2009) Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 110:469–485. doi:10.1111/j.1471-4159. 2009.06160.x
Al Sweidi S, Sánchez MG, Bourque M et al (2012) Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson’s disease. J Neuroendocrinol 24:48–61. doi:10.1111/j.1365-2826.2011.02193.x
Hamilton SR, Li H, Wischnewski H et al (2005) Intact {alpha}-1,2-endomannosidase is a typical type II membrane protein. Glycobiology 15:615–624
Fujishige K, Kotera J, Yuasa K, Omori K (2000) The human phosphodiesterase PDE10A gene genomic organization and evolutionary relatedness with other PDEs containing GAF domains. Eur J Biochem 267:5943–5951
Joset P, Wacker A, Babey R et al (2011) Rostral growth of commissural axons requires the cell adhesion molecule MDGA2. Neural Dev 6:22. doi:10.1186/1749-8104-6-22
Forno LS, DeLanney LE, Irwin I, Di Monte D et al (1992) Astrocytes and Parkinson’s disease. Prog Brain Res 94:429–436
Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I et al (1998) Microglial and astrocytic involvement in a murine model of Parkinson’s disease induced by 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP). Immunopharmacology 39:167–180
Chen X, Lan X, Roche I et al (2008) Caffeine protects against MPTP-induced blood–brain barrier dysfunction in mouse striatum. J Neurochem 107:1147–1157. doi:10.1111/j.1471- 4159.2008.05697.x
Kortekaas R, Leenders KL, van Oostrom JC et al (2005) Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57:176–179
Rite I, Machado A, Cano J, Venero JL (2007) Blood–brain barrier disruption induces in vivo degeneration of nigral dopaminergic neurons. J Neurochem 101:1567–1582
Hunot S, Hirsch EC (2003) Neuroinflammatory processes in Parkinson’s disease. Ann Neurol Suppl 3:S49–S58, discussion S58-60
Miklossy J, Doudet DD, Schwab C (2006) Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol 197:275–283
Bjursell M, Ahnmark A, Bohlooly YM (2007) Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56:583–593
Yamauchi T, Kamon J, Ito Y et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769
Huber JC, Schneeberger C, Tempfer CB (2002) Genetic modelling of the estrogen metabolism as a risk factor of hormone-dependent disorders. Maturitas 42:1–12
Zhang B, Liang C, Bates R et al (2012) Wnt proteins regulate acetylcholine receptor clustering in muscle cells. Mol Brain 5:7. doi:10.1186/1756-6606-5-7
Tanaka S, Terada K, Nohno T (2011) Canonical Wnt signaling is involved in switching from cell proliferation to myogenic differentiation of mouse myoblast cells. J Mol Signal 6:12. doi:10.1186/1750-2187-6-12
Reinhardt P, Schmid B, Burbulla LF et al (2013) Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 12:354–367. doi:10.1016/j.stem.2013.01.008
Matsushita Y, Oshima Y, Nakamura M (2007) Expression of DMRT genes in the gonads of Rana rugosa during sex determination. Zoolog Sci 24:95–99
Yoshizawa A, Nakahara Y, Izawa T et al (2011) Zebrafish Dmrta2 regulates neurogenesis in the telencephalon. Genes Cells 16:1097–1109. doi:10.1111/j.1365-2443.2011.01555.x
Endo M, Yasui K, Zen Y et al (2013) Alterations of the SWI/SNF chromatin remodelling subunit-BRG1 and BRM in hepatocellular carcinoma. Liver Int 33:105–117. doi:10.1111/ liv.12005
Wu Y, Yu L, Bi G et al (2003) Identification and characterization of two novel human SCAN domain-containing zinc finger genes ZNF396 and ZNF397. Gene 310:193–201. doi: 10.1002/humu.21067
Krejci P, Prochazkova J, Bryja V et al (2009) Molecular pathology of the fibroblast growth factor family. Hum Mutat 30:1245–1255. doi:10.1002/humu.21067
Makarenkova HP, Meech R (2012) Barx homeobox family in muscle development and regeneration. Int Rev Cell Mol Biol 297:117–173. doi:10.1016/B978-0-12-394308-8.00004 -2
Kraft P, Raychaudhuri S (2009) Complex diseases, complex genes: keeping pathways on the right track. Epidemiology 20:508–511. doi:10.1097/EDE.0b013e3181a93b98
Edwards YJ, Beecham GW, Scott WK et al (2011) Identifying consensus disease pathways in Parkinson’s disease using an integrative systems biology approach. PLoS One 6(e):16917. doi:10.1371/journal.pone.0016917
Peng G, Luo L, Siu H et al (2010) Gene and pathway-based second-wave analysis of genome-wide association studies. Eur J Hum Genet 18:111–117. doi:10.1038/ejhg.2009.115
Bossers K, Meerhoff G, Balesar R et al (2009) Analysis of gene expression in Parkinson’s disease: possible involvement of neurotrophic support and axon guidance in dopaminergic cell death. Brain Pathol 19:91–107. doi:10.1111/j.1750-3639.2008.00171.x
Sutherland GT, Matigian NA, Chalk AM et al (2009) A cross-study transcriptional analysis of Parkinson’s disease. PLoS One 4:e4955. doi:10.1371/journal.pone.0004955
Lin L, Lesnick TG, Maraganore DM, Isacson O (2009) Axon guidance and synaptic maintenance: preclinical markers for neurodegenerative disease and therapeutics. Trends Neurosci 32:142–149. doi:10.1016/j.tins.2008.11.006
Botta-Orfila T, Morató X, Compta Y et al (2014) Identification of blood serum micro-RNAs associated with idiopathic and LRRK2 Parkinson’s disease. J Neurosci Res 92:1071–1077. doi:10.1002/jnr.23377
Huang A, Martin ER, Vance JM, Cai X (2014) Detecting genetic interactions in pathway-based genome-wide association studies. Genet Epidemiol 38:300–309. doi:10.1002/gepi. 21803
Bednarczyk J, Lukasiuk K (2011) Tight junctions in neurological diseases. Acta Neurobiol Exp (Wars) 71:393–408
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. doi:10.1016/j.neuron.2008.01.003
Sung JY, Lee HJ, Jeong EI et al (2007) Alpha-synuclein overexpression reduces gap junctional intercellular communication in dopaminergic neuroblastoma cells. Neurosci Lett 416:289–293
Schwab BC, Heida T, Zhao Y et al (2014) Pallidal gap junctions-triggers of synchrony in Parkinson’s disease? Mov Disord 29:1486–1494. doi:10.1002/mds.25987
Acknowledgments
We are grateful to the PD patients who generously contributed their time and materials for this research.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This work was funded by the committee of the Chinese National Nature Science (grant number 30560042, 81160161, 81360198, 30871384), the education department of Jiangxi province (grant number [2005(183)], GJJ10303), and Guangdong Provincial Science & Technology Project Foundation (grant number 2012B031800410).
Author Contributions
Xu R, Deng L, and Zhang X conceived and designed the experiments. Hu Y, Zhang J, Deng L, Mei P, Wei Y, and Fang X performed the experiments. Xu R, Hu Y, Lin J, and Deng L analyzed the data. Cao X and Zhang X contributed partial materials. Xu R and Deng L wrote the paper. Hu Y, Deng L, and Zhang J are co-first authors. Xu R and Zhang X are the cooperation corresponding authors; these authors contributed to the research work of the same.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Hu, Y., Deng, L., Zhang, J. et al. A Pooling Genome-Wide Association Study Combining a Pathway Analysis for Typical Sporadic Parkinson’s Disease in the Han Population of Chinese Mainland. Mol Neurobiol 53, 4302–4318 (2016). https://doi.org/10.1007/s12035-015-9331-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9331-y