Abstract
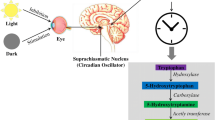
Melatonin is a neurohormone whose levels are significantly reduced or absent in Alzheimer’s disease (AD) patients. In these patients, acetylcholinesterase inhibitors (AChEI) are the major drug class used for their treatment; however, they present unwanted cholinergic side effects and have provided limited efficacy in clinic. Because combination therapy is being extensively used to treat different pathological diseases such as cancer or acquired immune deficiency syndrome, we posed this study to evaluate if melatonin in combination with an AChEI, galantamine, could provide beneficial properties in a novel in vitro model of AD. Thus, we subjected organotypic hippocampal cultures (OHCs) to subtoxic concentrations of β-amyloid (0.5 μM βA) plus okadaic acid (1 nM OA), for 4 days. This treatment increased by 95 % cell death, which was mainly apoptotic as shown by positive TUNEL staining. In addition, the combination of βA/OA increased Thioflavin S aggregates, hyperphosphorylation of Tau, oxidative stress (increased DCFDA fluorescence), and neuroinflammation (increased IL-1β and TNFα). Under these experimental conditions, melatonin (1–1000 nM) and galantamine (10–1000 nM), co-incubated with the toxic stimuli, caused a concentration-dependent neuroprotection; maximal neuroprotective effect was achieved at 1 μM of melatonin and galantamine. Most effective was the finding that combination of sub-effective concentrations of melatonin (1 nM) and galantamine (10 nM) provided a synergic anti-apoptotic effect and reduction of most of the AD-related pathological hallmarks observed in the βA/OA model. Therefore, we suggest that supplementation of melatonin in combination with lower doses of AChEIs could be an interesting strategy for AD patients.






Similar content being viewed by others
References
Duff K, Suleman F (2004) Transgenic mouse models of Alzheimer’s disease: how useful have they been for therapeutic development? Brief Funct Genom Proteomic 3(1):47–59
Hardy J (2002) Testing times for the amyloid cascade hypothesis. Neurobiol Aging 23(6):1073–1074
Avila J (2000) Tau aggregation into fibrillar polymers: taupathies. FEBS Lett 476(1-2):89–92
Bloom GS (2014) Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71(4):505–508. doi:10.1001/jamaneurol.2013.5847 1817720
Reiter RJ (1998) Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol 56(3):359–384
Leon R, Garcia AG, Marco-Contelles J (2013) Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med Res Rev 33(1):139–189. doi:10.1002/med.20248
Gareri P, Putignano D, Castagna A, Cotroneo AM, De Palo G, Fabbo A, Forgione L, Giacummo A et al (2014) Retrospective study on the benefits of combined Memantine and cholinEsterase inhibitor treatMent in AGEd Patients affected with Alzheimer’s Disease: the MEMAGE study. J Alzheimers Dis 41(2):633–640. doi:10.3233/JAD-132735
Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I (2004) Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 291(3):317–324. doi:10.1001/jama.291.3.317
Reiter RJ, Paredes SD, Manchester LC, Tan DX (2009) Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol 44(4):175–200. doi:10.1080/10409230903044914
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 51(1):1–16. doi:10.1111/j.1600-079X.2011.00916.x
Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res 54(3):245–257. doi:10.1111/jpi.12010
Gan L, Johnson JA (2014) Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta 1842(8):1208–1218. doi:10.1016/j.bbadis.2013.12.011
Rosales-Corral SA, Acuna-Castroviejo D, Coto-Montes A, Boga JA, Manchester LC, Fuentes-Broto L, Korkmaz A, Ma S et al (2012) Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res 52(2):167–202. doi:10.1111/j.1600-079X.2011.00937.x
Feng Y, Wang X (2012) Antioxidant therapies for Alzheimer’s disease. Oxid Med Cell Longev 2012:472932. doi:10.1155/2012/472932
Konrath EL, Passos Cdos S, Klein-Junior LC, Henriques AT (2013) Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J Pharm Pharmacol 65(12):1701–1725. doi:10.1111/jphp.12090
Nakamizo T, Kawamata J, Yamashita H, Kanki R, Kihara T, Sawada H, Akaike A, Shimohama S (2005) Stimulation of nicotinic acetylcholine receptors protects motor neurons. Biochem Biophys Res Commun 330(4):1285–1289. doi:10.1016/j.bbrc.2005.03.115
Park JE, Lee ST, Im WS, Chu K, Kim M (2008) Galantamine reduces striatal degeneration in 3-nitropropionic acid model of Huntington’s disease. Neurosci Lett 448(1):143–147. doi:10.1016/j.neulet.2008.10.020
Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez-Burillo S (2002) Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol 54(10):1299–1321. doi:10.1211/002235702760345374
Pappolla MA, Sos M, Omar RA, Bick RJ, Hickson-Bick DL, Reiter RJ, Efthimiopoulos S, Robakis NK (1997) Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J Neurosci: Off J Soc Neurosci 17(5):1683–1690
Yang X, Yang Y, Fu Z, Li Y, Feng J, Luo J, Zhang Q, Wang Q et al (2011) Melatonin ameliorates Alzheimer-like pathological changes and spatial memory retention impairment induced by calyculin A. J Psychopharmacol 25(8):1118–1125. doi:10.1177/0269881110367723
Iqbal K, Alonso AC, Gong CX, Khatoon S, Pei JJ, Wang JZ, Grundke-Iqbal I (1998) Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J Neural Transm Suppl 53:169–180
Buendia I, Egea J, Parada E, Navarro E, Leon R, Rodriguez-Franco MI, Lopez MG (2014) The melatonin-N, N-Dibenzyl(N-methyl)amine hybrid ITH91/IQM157 affords neuroprotection in an in vitro Alzheimer’s model via hemo-oxygenase-1 induction. ACS Chem Neurosci. doi:10.1021/cn5002073
Stoppini L, Parisi L, Oropesa C, Muller D (1997) Sprouting and functional recovery in co-cultures between old and young hippocampal organotypic slices. Neuroscience 80(4):1127–1136
Ha HC, Woster PM, Yager JD, Casero RA Jr (1997) The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci U S A 94(21):11557–11562
Wu YH, Swaab DF (2005) The human pineal gland and melatonin in aging and Alzheimer’s disease. J Pineal Res 38(3):145–152. doi:10.1111/j.1600-079X.2004.00196.x
Iqbal K, Grundke-Iqbal I (2000) Alzheimer disease is multifactorial and heterogeneous. Neurobiol Aging 21(6):901–902, discussion 903-904
Marlatt MW, Bauer J, Aronica E, van Haastert ES, Hoozemans JJ, Joels M, Lucassen PJ (2014) Proliferation in the Alzheimer hippocampus is due to microglia, not astroglia, and occurs at sites of amyloid deposition. Neural Plast 2014:693851. doi:10.1155/2014/693851
Egea J, Martin-de-Saavedra MD, Parada E, Romero A, Del Barrio L, Rosa AO, Garcia AG, Lopez MG (2012) Galantamine elicits neuroprotection by inhibiting iNOS, NADPH oxidase and ROS in hippocampal slices stressed with anoxia/reoxygenation. Neuropharmacology 62(2):1082–1090. doi:10.1016/j.neuropharm.2011.10.022
Lorrio S, Sobrado M, Arias E, Roda JM, Garcia AG, Lopez MG (2007) Galantamine postischemia provides neuroprotection and memory recovery against transient global cerebral ischemia in gerbils. J Pharmacol Exp Ther 322(2):591–599. doi:10.1124/jpet.107.122747
Nassif M, Hoppe J, Santin K, Frozza R, Zamin LL, Simao F, Horn AP, Salbego C (2007) Beta-amyloid peptide toxicity in organotypic hippocampal slice culture involves Akt/PKB, GSK-3beta, and PTEN. Neurochem Int 50(1):229–235. doi:10.1016/j.neuint.2006.08.008
Hoppe JB, Frozza RL, Horn AP, Comiran RA, Bernardi A, Campos MM, Battastini AM, Salbego C (2010) Amyloid-beta neurotoxicity in organotypic culture is attenuated by melatonin: involvement of GSK-3beta, tau and neuroinflammation. J Pineal Res 48(3):230–238. doi:10.1111/j.1600-079X.2010.00747.x
Frozza RL, Horn AP, Hoppe JB, Simao F, Gerhardt D, Comiran RA, Salbego CG (2009) A comparative study of beta-amyloid peptides Abeta1-42 and Abeta25-35 toxicity in organotypic hippocampal slice cultures. Neurochem Res 34(2):295–303. doi:10.1007/s11064-008-9776-8
Lassmann H, Bancher C, Breitschopf H, Wegiel J, Bobinski M, Jellinger K, Wisniewski HM (1995) Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathol 89(1):35–41
Li WP, Chan WY, Lai HW, Yew DT (1997) Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J Mol Neurosci 8(2):75–82. doi:10.1007/BF02736774
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H et al (2009) Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A 106(47):20057–20062. doi:10.1073/pnas.0905529106
Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC et al (2014) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 41(2):615–631. doi:10.3233/JAD-132690
Hansen RA, Gartlehner G, Lohr KN, Kaufer DI (2007) Functional outcomes of drug treatment in Alzheimer’s disease: a systematic review and meta-analysis. Drugs Aging 24(2):155–167
Traykova M, Traykov T, Hadjimitova V, Krikorian K, Bojadgieva N (2003) Antioxidant properties of galantamine hydrobromide. Z Naturforsch C 58(5-6):361–365
Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, Wang L, Zhang C et al (2009) Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res 47(1):82–96. doi:10.1111/j.1600-079X.2009.00692.x
Slats D, Claassen JA, Verbeek MM, Overeem S (2013) Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: focus on the role of hypocretin and melatonin. Ageing Res Rev 12(1):188–200. doi:10.1016/j.arr.2012.04.003
Kvetnoy IM (1999) Extrapineal melatonin: location and role within diffuse neuroendocrine system. Histochem J 31(1):1–12
Reiter RJ (1994) Pineal function during aging: attenuation of the melatonin rhythm and its neurobiological consequences. Acta Neurobiol Exp (Wars) 54(Suppl):31–39
Srinivasan V, Maestroni GJ, Cardinali DP, Esquifino AI, Perumal SR, Miller SC (2005) Melatonin, immune function and aging. Immun Ageing 2:17. doi:10.1186/1742-4933-2-17
Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P (2012) The role of melatonin in acute myocardial infarction. Front Biosci (Landmark Ed) 17:2433–2441
Hardeland R (2009) Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 35(2):183–192. doi:10.1002/biof.23
Acuna Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ (2011) Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem 11(2):221–240
Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, Frydman-Marom A, Zisapel N (2014) Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging 9:947–961. doi:10.2147/CIA.S65625
Acknowledgments
This work was supported by the Spanish Ministry of Economy and Competence Ref. SAF2012-32223 to MGL. IB has a predoctoral FPU fellowship from the Spanish Ministry of Economy and Competence (AP2010/1219), EP has a predoctoral FPI fellowship from the Spanish Ministry of Economy and Competence, and EN (FPI-UAM2012) from Universidad Autónoma de Madrid. IS Carlos III, Programa Miguel Servet (CP11/00165) and FIS proyect (grant PI14/00372) and European Commission, Marie Curie Actions FP7 (FP7-People-2012-CIG-322156) to RL. JE has a grant from IS Carlos III, Programa Miguel Servet (CP14/00008) and IS Carlos III research contract under Miguel Servet Program. We would like to thank Vanessa Gómez Rangel for technical support, Mª Dolores Morales García and Ana Isabel de las Heras Núñez from the Confocal Service of de Universidad Autónoma de Madrid. We also thank the continuous support of Fundación Teófilo Hernando.
Compliance with Ethical Standards
All animal assays were carried out following the Guide for the Care and Use of Laboratory Animals and were previously approved by the Institutional Ethics Committee of the Autonomous University of Madrid, Spain, according to the European guidelines for the use and care of animals for research in accordance with the European Union Directive of 22 September 2010 (2010/63/UE) and with the Spanish Royal Decree of 1 February 2013 (53/2013). All efforts were made to minimize the number of animals used and their suffering.
Conflict of Interest
All authors have no conflict of interest.
Author Contributions
Izaskun Buendia has contributed to acquisition of data, data analysis/interpretation, writing, and critical revision of the manuscript. Esther Parada has contributed to acquisition of data and data analysis/interpretation. Elisa Navarro has contributed to acquisition of data and data analysis/interpretation. Rafael León has contributed to critical revision of the manuscript. Pilar Negredo has contributed to critical revision of the manuscript. Javier Egea has contributed to concept/design, acquisition of data, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript, and approval of the article. Manuela García López has contributed to concept/design, drafting of the manuscript, critical revision of the manuscript, and approval of the article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
I. Buendia and E. Parada contributed equally to this work.
Rights and permissions
About this article
Cite this article
Buendia, I., Parada, E., Navarro, E. et al. Subthreshold Concentrations of Melatonin and Galantamine Improves Pathological AD-Hallmarks in Hippocampal Organotypic Cultures. Mol Neurobiol 53, 3338–3348 (2016). https://doi.org/10.1007/s12035-015-9272-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9272-5