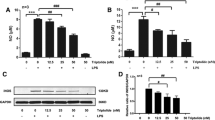
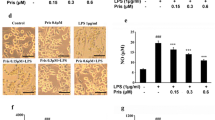
Abstract
Regulatory mechanisms of the expression of interleukin-10 (IL-10) in brain inflammatory conditions remain elusive. To address this issue, we used multiple primary brain cell cultures to study the expression of IL-10 in lipopolysaccharide (LPS)-elicited inflammatory conditions. In neuron–glia cultures, LPS triggered well-orchestrated expression of various immune factors in the following order: tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and lastly IL-10, and these inflammatory mediators were mainly produced from microglia. While exogenous application of individual earlier-released pro-inflammatory factors (e.g., TNF-α, IL-1β, or PGE2) failed to induce IL-10 expression, removal of LPS from the cultures showed the requirement of continuing presence of LPS for IL-10 expression. Interestingly, genetic disruption of tnf-α, its receptors tnf-r1/r2, and cox-2 and pharmacological inhibition of COX-2 activity enhanced LPS-induced IL-10 production in microglia, which suggests negative regulation of IL-10 induction by the earlier-released TNF-α and PGE2. Further studies showed that negative regulation of IL-10 production by TNF-α is mediated by PGE2. Mechanistic studies indicated that PGE2-elicited suppression of IL-10 induction was eliminated by genetic disruption of the PGE2 receptor EP2 and was mimicked by the specific agonist for the EP2, butaprost, but not agonists for the other three EP receptors. Inhibition of cAMP-dependent signal transduction failed to affect PGE2-mediated inhibition of IL-10 production, suggesting that a G protein-independent pathway was involved. Indeed, deficiency in β-arrestin-1 or β-arrestin-2 abolished PGE2-elicited suppression of IL-10 production. In conclusion, we have demonstrated that COX-2-derived PGE2 inhibits IL-10 expression in brain microglia through a novel EP2- and β-arrestin-dependent signaling pathway.






Similar content being viewed by others
References
Merrill JE, Benveniste EN (1996) Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 19(8):331–338
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454(7203):428–435. doi:10.1038/nature07201
Nathan C (2002) Points of control in inflammation. Nature 420(6917):846–852. doi:10.1038/nature01320
Kielian T (2006) Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res 83(5):711–730. doi:10.1002/jnr.20767
Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8(5):349–361. doi:10.1038/nri2294
Gao HM, Hong JS (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29(8):357–365
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55(5):453–462. doi:10.1002/glia.20467
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47. doi:10.1186/1750-1326-4-47
Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW (2001) Interleukin-10 in the brain. Crit Rev Immunol 21(5):427–449
Saraiva M, O'Garra A (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10(3):170–181. doi:10.1038/nri2711
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75(2):263–274
Heyen JR, Ye S, Finck BN, Johnson RW (2000) Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kappaB. Brain Res Mol Brain Res 77(1):138–147
Kremlev SG, Palmer C (2005) Interleukin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. J Neuroimmunol 162(1–2):71–80. doi:10.1016/j.jneuroim.2005.01.010
Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J (2010) Biology of interleukin-10. Cytokine Growth Factor Rev 21(5):331–344. doi:10.1016/j.cytogfr.2010.09.002
Qian L, Block ML, Wei SJ, Lin CF, Reece J, Pang H, Wilson B, Hong JS, Flood PM (2006) Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther 319(1):44–52. doi:10.1124/jpet.106.106351
Sheng WS, Hu S, Kravitz FH, Peterson PK, Chao CC (1995) Tumor necrosis factor alpha upregulates human microglial cell production of interleukin-10 in vitro. Clin Diagn Lab Immunol 2(5):604–608
Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BM, Brennan FM (1998) Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol 160(2):920–928
Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H (2000) IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol 165(1):286–291
Platzer C, Meisel C, Vogt K, Platzer M, Volk HD (1995) Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol 7(4):517–523
Harizi H, Gualde N (2006) Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell Mol Immunol 3(4):271–277
Aloisi F, De Simone R, Columba-Cabezas S, Levi G (1999) Opposite effects of interferon-gamma and prostaglandin E2 on tumor necrosis factor and interleukin-10 production in microglia: a regulatory loop controlling microglia pro- and anti-inflammatory activities. J Neurosci Res 56(6):571–580
Seo DR, Kim KY, Lee YB (2004) Interleukin-10 expression in lipopolysaccharide-activated microglia is mediated by extracellular ATP in an autocrine fashion. Neuroreport 15(7):1157–1161
Cheon H, Rho YH, Choi SJ, Lee YH, Song GG, Sohn J, Won NH, Ji JD (2006) Prostaglandin E2 augments IL-10 signaling and function. J Immunol 177(2):1092–1100
Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X (2007) Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27(6):952–964. doi:10.1016/j.immuni.2007.11.014
Koscso B, Csoka B, Kokai E, Nemeth ZH, Pacher P, Virag L, Leibovich SJ, Hasko G (2013) Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol. doi:10.1189/jlb.0113043
Chang EY, Guo B, Doyle SE, Cheng G (2007) Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol 178(11):6705–6709
Quan Y, Jiang J, Dingledine R (2013) EP2 receptor signaling pathways regulate classical activation of microglia. J Biol Chem 288(13):9293–9302
Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal MR (2009) Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med 206(3):535–548. doi:10.1084/jem.20082293
Chun KS, Lao HC, Langenbach R (2010) The prostaglandin E2 receptor, EP2, stimulates keratinocyte proliferation in mouse skin by G protein-dependent and {beta}-arrestin1-dependent signaling pathways. J Biol Chem 285(51):39672–39681. doi:10.1074/jbc.M110.117689
Trivedi DB, Loftin CD, Clark J, Myers P, DeGraff LM, Cheng J, Zeldin DC, Langenbach R (2013) Beta-arrestin-2 deficiency attenuates abdominal aortic aneurysm formation in mice. Circ Res 112(9):1219–1229. doi:10.1161/CIRCRESAHA.112.280399
Chen SH, Oyarzabal EA, Hong JS (2013) Preparation of rodent primary cultures for neuron-glia, mixed glia, enriched microglia, and reconstituted cultures with microglia. Methods Mol Biol 1041:231–240. doi:10.1007/978-1-62703-520-0_21
Zhang SC, Fedoroff S (1996) Neuron-microglia interactions in vitro. Acta Neuropathol 91(4):385–395
Gebicke-Haerter PJ, Bauer J, Schobert A, Northoff H (1989) Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci Off J Soc Neurosci 9(1):183–194
Kambayashi Y, Takekoshi S, Tanino Y, Watanabe K, Nakano M, Hitomi Y, Takigawa T, Ogino K, Yamamoto Y (2007) Various molecular species of diacylglycerol hydroperoxide activate human neutrophils via PKC activation. J Clin Biochem Nutr 41(1):68–75. doi:10.3164/jcbn.2007009
Karlsson A, Nixon JB, McPhail LC (2000) Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: dependent or independent of phosphatidylinositol 3-kinase. J Leukoc Biol 67(3):396–404
Evans ME, Feola DJ, Rapp RP (1999) Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother 33(9):960–967
Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Hasko G (2007) A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 110(7):2685–2695. doi:10.1182/blood-2007-01-065870
Wong KF, Luk JM, Cheng RH, Klickstein LB, Fan ST (2007) Characterization of two novel LPS-binding sites in leukocyte integrin beta A domain. FASEB J 21(12):3231–3239. doi:10.1096/fj.06-7579com
Eissner G, Kirchner S, Lindner H, Kolch W, Janosch P, Grell M, Scheurich P, Andreesen R, Holler E (2000) Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages. J Immunol 164(12):6193–6198
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188(1):21–28. doi:10.4049/jimmunol.1101029
Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by beta-arrestins. Science 308(5721):512–517. doi:10.1126/science.1109237
Fan XL, Zhang JS, Zhang XQ, Yue W, Ma L (2003) Differential regulation of beta-arrestin 1 and beta-arrestin 2 gene expression in rat brain by morphine. Neuroscience 117(2):383–389
Qian L, Wu HM, Chen SH, Zhang D, Ali SF, Peterson L, Wilson B, Lu RB, Hong JS, Flood PM (2011) Beta2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway. J Immunol 186(7):4443–4454. doi:10.4049/jimmunol.1002449
Straccia M, Gresa-Arribas N, Dentesano G, Ejarque-Ortiz A, Tusell JM, Serratosa J, Sola C, Saura J (2011) Pro-inflammatory gene expression and neurotoxic effects of activated microglia are attenuated by absence of CCAAT/enhancer binding protein beta. J Neuroinflammation 8:156. doi:10.1186/1742-2094-8-156
Biswas SK, Lopez-Collazo E (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30(10):475–487. doi:10.1016/j.it.2009.07.009
Shirai Y, Hashimoto M, Kato R, Kawamura YI, Kirikae T, Yano H, Takashima J, Kirihara Y, Saito Y, Fujino MA, Dohi T (2004) Lipopolysaccharide induces CD25-positive, IL-10-producing lymphocytes without secretion of proinflammatory cytokines in the human colon: low MD-2 mRNA expression in colonic macrophages. J Clin Immunol 24(1):42–52. doi:10.1023/B:JOCI.0000018062.01980.ba
Johansson JU, Pradhan S, Lokteva LA, Woodling NS, Ko N, Brown HD, Wang Q, Loh C, Cekanaviciute E, Buckwalter M, Manning-Bog AB, Andreasson KI (2013) Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. J Neurosci Off J Soc Neurosci 33(40):16016–16032. doi:10.1523/JNEUROSCI.2203-13.2013
Chun KS, Lao HC, Trempus CS, Okada M, Langenbach R (2009) The prostaglandin receptor EP2 activates multiple signaling pathways and beta-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis 30(9):1620–1627
Yun SP, Ryu JM, Jang MW, Han HJ (2011) Interaction of profilin-1 and F-actin via a beta-arrestin-1/JNK signaling pathway involved in prostaglandin E(2)-induced human mesenchymal stem cells migration and proliferation. J Cell Physiol 226(2):559–571. doi:10.1002/jcp.22366
Acknowledgments
This work was supported in part by the Intramural Research Program of the NIH/NIEHS (ES090082; ES025043), the National Natural Science Foundation of China, and the award to high-level innovative and entrepreneurial talents of Jiangsu Province of China. We thank Anthony Lockhart for the assistance with animal colony management and maintenance of the timed pregnant mice.
Conflict of Interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chun-Hsien Chu and Shih-Heng Chen contributed equally to this paper.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Effects of polymyxin B on neuron-glia cultures treated with LPS. A. Immunostaining of neuron-glia cells treated with LPS (15 ng/ml) and polymyxin B (10 μg/ml) at 48 hours using anti-Iba-1 antibody. Iba-1 served as a microglia marker. B. [3H] dopamine uptake of neuron-glia cells treated with LPS (15 ng/ml) and polymyxin B (10 μg/ml) at 7 days. Results of dopamine uptake were represented as percentage of vehicle-treated control cultures. Data were expressed as means ± SEM from three independent experiments run in triplicate. *, p < 0.05, compared with corresponding vehicle-treated control cultures. #, p < 0.05, compared with corresponding LPS-treated cultures. Magnification: 200X (GIF 71 kb)
Supplemental Fig. 2
Both TLR4 and Mac-1 receptors participate in induction of IL-10 by LPS. Mixed-glia cultures from wildtype (FeJ) and TLR4-deficient (HeJ) mice (A) or from wildtype and Mac-1-deficient mice (B) were treated with LPS (15 ng/ml). IL-10 production in the supernatant of these cells was measured at 48 hours following LPS treatment by ELISA. Results are shown as the mean ± SEM from 3 independent experiments. *, p < 0.05, compared with vehicle-treated control cultures. #, p < 0.05, compared with corresponding LPS-treated wildtype cultures. C. LPS induces IL-10 production through MAPK and NF-κB signaling pathways. Neuron-glia cultures were pre-treated with a variety of protein kinase inhibitors including SP6000125 (5 μM; for JNK), SB203580 (1 μM; for p38), U0126 (10 μM; for ERK), Bay 11-7821 (10 μM; for NF-κB), Rp-cAMPs (10 μM; for PKA) and Calphostin C (1 μM; for PKC) for 1 hour, and then exposure to LPS (15 ng/ml). IL-10 production in the supernatant of these cells was measured at 48 hours following LPS treatment by ELISA. Results are represented as percentage of LPS-treated group and shown as the mean ± SEM from 3 independent experiments. *, p < 0.05, compared with corresponding LPS-treated wildtype cultures. (GIF 24 kb)
Supplemental Fig. 3
Deficiency in tnf-α or its receptor genes reduces LPS-induced pro-inflammatory factors in microglia. A. Mixed-glia cultures were prepared from wildtype, TNF-α-deficient, or TNF-R1/R2-deficient mice. After 2 weeks of cells seeding, immunocytochemical analysis of microglia in these mixed-glia cultures was performed by using anti-Iba-1 antibody. B. After 3 hours of LPS treatment (15 ng/ml), TNF-α secretion into the supernatant of mixed-glia cultures was detected by ELISA. Results are shown as the mean ± SEM from 3 independent experiments. *, p < 0.05, compared with vehicle-treated control cultures. #, p < 0.05, compared with corresponding LPS-treated wildtype cultures. C. Expression of COX-2 and iNOS mRNA in mixed-glia cultures at 6 hours after LPS treatment was measured by RT-PCR. Results are represented as percentage of LPS-treated group and shown as the mean ± SEM from 3 independent experiments. #, p < 0.05, compared with corresponding LPS-treated wildtype cultures. (GIF 108 kb)
Supplemental Fig. 4
LPS induces similar amount of TNF-α production in wildtype and COX-2-deficient mixed-glia cultures. Three hours after these cultures were treated with LPS (15 ng/ml), TNF-α secretion into the supernatant of these cultures was detected by ELISA. Results are shown as the mean ± SEM from 3 independent experiments. *, p < 0.05, compared with vehicle-treated control cultures. (GIF 6 kb)
Supplemental Fig. 5
Protein kinase inhibition fails to restore reduction of IL-10 release by PGE2. PGE2 and protein kinase inhibitors including wortmannin (50 nM; for PI3K) (A), SB216763 (1 μM; for GSK3β) (B), U0126 (10 μM; for ERK) (C), SP600125 (5 μM; for JNK) (D), and PD98059 (50 μM; for MEK1/2) (E) were added into neuron-glia cultures after these cultures were treated with LPS for 24 hours. IL-10 release into the supernatants was detected at 24 hours following PGE2 treatment (48 hours after LPS treatment) by ELISA. The experiment has been performed three times. Results are shown as the mean ± SEM. *, p < 0.05, compared with corresponding vehicle-treated control cultures; #, p < 0.05 relative to corresponding LPS-treated cultures. NS: non-significant. (GIF 73 kb)
Supplemental Fig. 6
LPS induces similar amount of TNF-α production in wildtype, β-arrestin-1- deficient, and β-arrestin-2- deficient mixed-glia cultures. Three hours after these cultures were treated with LPS (15 ng/ml), TNF-α secretion into the supernatant of these cells were detected by ELISA. Results are shown as the mean ± SEM from 3 independent experiments. *, p < 0.05, compared with vehicle-treated control cultures. (GIF 9 kb)
Rights and permissions
About this article
Cite this article
Chu, CH., Chen, SH., Wang, Q. et al. PGE2 Inhibits IL-10 Production via EP2-Mediated β-Arrestin Signaling in Neuroinflammatory Condition. Mol Neurobiol 52, 587–600 (2015). https://doi.org/10.1007/s12035-014-8889-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8889-0