Abstract
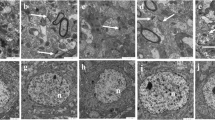
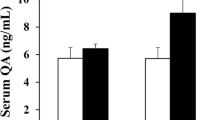
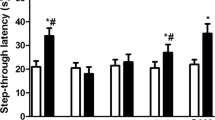
Realgar is a type of mineral drug containing arsenic. The nervous system toxicity of realgar has received extensive attention. However, the underlying mechanisms of realgar-induced neurotoxicity have not been clearly elucidated. To explore the mechanisms that contribute to realgar-induced neurotoxicity, weanling rats were exposed to realgar (0, 0.3, 0.9, 2.7 g/kg) for 6 weeks, and cognitive ability was tested using the Morris water maze (MWM) test and object recognition task (ORT). The levels of arsenic in the blood and hippocampus were monitored. The ultrastructures of hippocampal neurons were observed. The levels of glutamate (Glu) and glutamine (Gln) in the hippocampus and hippocampal CA1 region; the activities of glutamine synthetase (GS) and phosphate-activated glutaminase (PAG); the mRNA and protein expression of glutamate transporter 1 (GLT-1), glutamate/aspartate transporter (GLAST), and N-methyl-d-aspartate (NMDA) receptors; and the level of intracellular Ca2+ were also investigated. The results indicate that the rats developed deficiencies in cognitive ability after a 6-week exposure to realgar. The arsenic contained in realgar and the arsenic metabolites passed through the blood-brain barrier (BBB) and accumulated in the hippocampus, which resulted in the excessive accumulation of Glu in the extracellular space. The excessive accumulation of Glu in the extracellular space induced excitotoxicity, which was shown by enhanced GS and PAG activities, inhibition of GLT-1 mRNA and protein expression, alterations in NMDA receptor mRNA and protein expression, disturbance of intracellular Ca2+ homeostasis, and ultrastructural changes in hippocampal neurons. In conclusion, the findings from our study indicate that exposure to realgar induces excitotoxicity and that the mechanism by which this occurs may be associated with disturbances in Glu metabolism and transportation and alterations in NMDA receptor expression.










Similar content being viewed by others
Abbreviations
- TCMs:
-
Traditional Chinese medicines
- BBB:
-
Blood-brain barrier
- MWM:
-
Morris water maze
- ORT:
-
Object recognition task
- Glu:
-
Glutamate
- CNS:
-
Central nervous system
- GLT-1:
-
Glutamate transporter 1
- GLAST:
-
Glutamate/aspartate transporter
- GS:
-
Glutamine synthetase
- Gln:
-
Glutamine
- PAG:
-
Phosphate-activated glutaminase
- NMDA:
-
N-Methyl-d-aspartate
- iAs:
-
Inorganic arsenic
- MMA:
-
Methyl arsine
- DMA:
-
Dimethyl arsine
- ECL:
-
Enhanced chemiluminescence
- CMC-Na:
-
Sodium carboxymethylcellulose
- MD:
-
Microdialysis
- NE:
-
Northeast
- NW:
-
Northwest
- SE:
-
Southeast
- SW:
-
Southwest
- AAS:
-
Atomic absorption spectrophotometry
- TAs:
-
Total arsenic
- HPLC:
-
High-performance liquid chromatography
- PVDF:
-
Polyvinylidene difluoride
- TBS:
-
Tris-buffered saline
- PBS:
-
Phosphate-buffered saline
- NOAEL:
-
No observed adverse effect level
References
Chinese Pharmacopeia Committee (2010) Pharmacopoeia of China. People's Press, Beijing, p 316
Liang A, Li C, Wang J et al (2011) Toxicity study of realgar. Zhongguo Zhong Yao Za Zhi 36:1889–1894
Zhang L, Gao S, Zhou C et al (2006) Safety issues of traditional Chinese medical preparations containing arsenic substances: review starting from Niuhuang Jiedu Pian (Wan). Zhongguo Zhong Yao Za Zhi 31:2010–2013
Vahidnia A, van der Voet GB, de Wolff FA (2007) Arsenic neurotoxicity—a review. Hum Exp Toxicol 26:823–832
Ehrenstein OS, Poddar S, Yuan Y et al (2007) Children’s intellectual function in relation to arsenic exposure. Epidemiology 18:44–51
Roy A, Kordas K, Lopez P et al (2011) Association between arsenic exposure and behavior among first-graders from Torreón, Mexico. Environ Res 111:670–676
Zhang Y, Qiang S, Sun J et al (2013) Liquid chromatography-hydride generation-atomic fluorescence spectrometry determination of arsenic species in dog plasma and its application to a pharmacokinetic study after oral administration of Realgar and Niu Huang Jie Du Pian. J Chromatogr B 917–918:93–99
Tang Y, Wang N, Mi S et al (2009) Excretion of arsenic in realgar and its metabolites in rats. Pharmacol Clin Chin Mater Med 25:39–42
Li C, Liang A, Wang J et al (2011) Arsenic accumulation following realgar administration in rats. China J Chin Mater Med 36:1895–1899
Huo T, Chang B, Zhang Y et al (2012) Alteration of amino acid neurotransmitters in brain tissues of immature rats treated with realgar. J Pharm Biomed Anal 57:120–124
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. Neurosci Methods 11:47–60
Akkerman S, Prickaerts J, Steinbusch HW et al (2012) Object recognition testing: statistical considerations. Behav Brain Res 232:317–322
Burbaeva GS, Boksha IS, Tereshkina EB et al (2005) Glutamate metabolizing enzymes in prefrontal cortex of Alzheimer’s disease patients. Neurochem Res 30:1443–1451
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Heyser CJ, Ferris JS (2013) Object exploration in the developing rat: methodological considerations. Dev Psychobiol 55:373–381
Renis M, Cardile V, Russo A et al (1998) Glutamine synthetase activity and HSP70 levels in cultured rat astrocytes: effects of 1-octadecyl-2-methyl-rac-glycero-3-phospho-choline. Brain Res 783:143–150
Curi TC, De Melo MP, De Azevedo RB et al (1997) Glutamine utilization by rat neurophils: presence of phosphate-dependent glutaminase. Am J Physiol 273:1124–1129
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
van Goethem NP, Rutten K, van der Staay FJ et al (2012) Object recognition testing: rodent species, strains, housing conditions, and estrous cycle. Behav Brain Res 232:323–334
Rodríguez VM, Del Razo LM, Limon-Pacheco JH et al (2005) Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol Sci 84:157–166
Majumdar KK, Guha Mazumder DN (2012) Effect of drinking arsenic-contaminated water in children. Indian J Public Health 56:223–226
Rodríguez VM, Carrizales L, Jiménez-Capdeville ME et al (2001) The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull 55:301–308
Rodríguez VM, Carrizales L, Mendoza MS et al (2002) Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol 24:743–750
Luo J, Qiu Z, Shu W et al (2009) Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol Lett 184:121–125
Qu C, Niu Y, Zhong Y et al (2007) Effects of subchronic arsenic exposure on glutamate-glutamine cycle in mice brain. J Environ Health 24:751–754
Zhao F, Liao Y, Jin Y et al (2012) Effect of arsenite on glutamate metabolism in primary cultured astrocytes. Toxicol in Vitro 26:24–31
Shachnai L, Shimamoto K, Kanner BI (2005) Sulfhydryl modification of cysteine mutants of a neuronal glutamate transporter reveals an inverse relationship between sodium dependent conformational changes and the glutamate-gated anion conductance. Neuropharmacology 49:862–871
Lehre KP, Danbolt NC (1998) The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 18:8751–8757
Gazzaley AH, Weiland NG, McEwen BS et al (1996) Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci 16:6830–6838
Follesa P, Ticku MK (1995) Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Mol Brain Res 29:99–106
Follesa P, Ticku MK (1996) NMDA receptor upregulation: molecular studies in cultured mouse cortical neurons after chronic antagonist exposure. J Neurosci 16:2172–2178
Trevisan L, Fitzgerald LW, Brose N et al (1994) Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem 62:1635–1638
Luo J, Qiu Z, Zhang L et al (2012) Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol Lett 11:39–44
Pizzi M, Consolandi O, Memo M et al (1996) Activation of multiple metabotropic glutamate receptor subtypes prevents NMDA-induced excitotoxicity in rat hippocampal slices. Eur J Neurosci 8:1516–1521
Muscoli C, Visalli V, Colica C et al (2005) The effect of inflammatory stimuli on NMDA-related activation of glutamine synthase in human cultured astroglial cells. Neurosci Lett 373:184–188
Caballero-Benitez A, Alavez S, Uribe RM et al (2004) Regulation of glutamate-synthesizing enzymes by NMDA and potassium in cerebellar granule cells. Eur J Neurosci 19:2030–2038
Acknowledgments
The work is financially supported by the National Natural Science Foundation of China (81073144), the China Postdoctoral Science Foundation on the 51th grant program (2012M510848), and the Higher Education Department of Liaoning Province (L2010708).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huo, Tg., Li, Wk., Zhang, Yh. et al. Excitotoxicity Induced by Realgar in the Rat Hippocampus: the Involvement of Learning Memory Injury, Dysfunction of Glutamate Metabolism and NMDA Receptors. Mol Neurobiol 51, 980–994 (2015). https://doi.org/10.1007/s12035-014-8753-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8753-2