Abstract
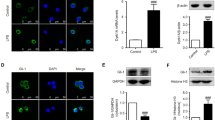
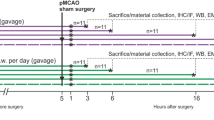
Neuroinflammation is important for the development of several neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and stroke. Since changes of cytokine level are critical for neuroinflammation in the brain, we investigated whether IL-32α overexpression could change neuroinflammation and, thus, affect stroke development. Middle cerebral artery occlusion (MCAO) induced development of ischemia, and ischemic neuronal cell death were reduced in IL-32α-overexpressing transgenic mice (IL-32α mice) brain through the decreased release of neuroinflammatory cytokines (IL-6, IL-1β, TNF-α) and activation of astrocytes, but enhancement of anti-neuroinflammatory cytokines (IL-10). Reactive oxygen species generation and lipid peroxidation as well as expression of inducible nitric oxide and cyclooxygenase-2 were also reduced in the IL-32α mice brain. Nuclear factor-kappa B (NF-κB), a critical transcriptional factor regulating neuroinflammation, was much lower, but activation of signal transducer and activator of transcription 3 (STAT3), which plays a crucial role in cell survival and proliferation, was much higher in IL-32α-overexpressing mice brain compared to those of wild-type mice brain. These results suggest that IL-32α can prevent cerebral ischemia damage via upregulation of anti-neuroinflammatory cytokine expression and STAT3 activation, but downregulation of neuroinflammatory cytokines and NF-κB activation.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- COX-2:
-
Cyclooxygenase-2
- DCM:
-
Dichloromethane
- ELISA:
-
Enzyme-linked immunosorbent assay
- EMSA:
-
Gel electromobility shift assay
- GFAP:
-
Glial fibrillary acidic protein
- IL:
-
Interleukin
- IκB:
-
Inhibitor of κB
- iNOS:
-
Inducible nitric oxide synthase
- KO:
-
Knockout
- MCAO:
-
Middle cerebral artery occlusion
- NF-κB:
-
Nuclear factor-kappa B
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- STAT:
-
Signal transducer and activator of transcription
- TNF:
-
Tumor necrosis factor
- TTC:
-
2,3,5-Triphenyltetrazolium chloride.
References
Chen YC, Wu JS, Yang ST, Huang CY, Chang C, Sun GY, Lin TN (2012) Stroke, angiogenesis and phytochemicals. Front Biosci (Schol Ed) 4:599–610
Li X, Luo P, Wang Q, Xiong L (2012) Electroacupuncture pretreatment as a novel avenue to protect brain against ischemia and reperfusion injury. Evid Based Complement Alternat Med 2012:195397. doi:10.1155/2012/195397
Dong L, Qiao H, Zhang X, Zhang X, Wang C, Wang L, Cui L, Zhao J, Xing Y, Li Y, Liu Z, Zhu C (2013) Parthenolide is neuroprotective in rat experimental stroke model: downregulating NF-kappaB, phospho-p38MAPK, and caspase-1 and ameliorating BBB permeability. Mediators Inflamm 2013:370804. doi:10.1155/2013/370804
Kang JW, Choi SC, Cho MC, Kim HJ, Kim JH, Lim JS, Kim SH, Han JY, Yoon DY (2009) A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology 128(1 Suppl):e532–540. doi:10.1111/j.1365-2567.2008.03025.x
Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA (2005) Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 22(1):131–142. doi:10.1016/j.immuni.2004.12.003
Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA, Kim SH (2005) IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A 102(45):16309–16314. doi:10.1073/pnas.0508237102
Oh JH, Cho MC, Kim JH, Lee SY, Kim HJ, Park ES, Ban JO, Kang JW, Lee DH, Shim JH, Han SB, Moon DC, Park YH, Yu DY, Kim JM, Kim SH, Yoon DY, Hong JT (2011) IL-32gamma inhibits cancer cell growth through inactivation of NF-kappaB and STAT3 signals. Oncogene 30(30):3345–3359. doi:10.1038/onc.2011.52
Rowinsky EK, Onetto N, Canetta RM, Arbuck SG (1992) Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol 19(6):646–662
Choi J, Bae S, Hong J, Ryoo S, Jhun H, Hong K, Yoon D, Lee S, Her E, Choi W, Kim J, Azam T, Dinarello CA, Kim S (2010) Paradoxical effects of constitutive human IL-32{gamma} in transgenic mice during experimental colitis. Proc Natl Acad Sci U S A 107(49):21082–21086. doi:10.1073/pnas.1015418107
Geronikaki AA, Gavalas AM (2006) Antioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen 9(6):425–442
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10(8):1343–1374. doi:10.1089/ars.2007.1957
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21(1):2–14. doi:10.1097/00004647-200101000-00002
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4(5):399–415. doi:10.1038/nrn1106
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262(5134):689–695
Krapfenbauer K, Yoo BC, Fountoulakis M, Mitrova E, Lubec G (2002) Expression patterns of antioxidant proteins in brains of patients with sporadic Creutzfeldt-Jacob disease. Electrophoresis 23(15):2541–2547. doi:10.1002/1522-2683(200208)23:15<2541::AID-ELPS2541>3.0.CO;2-1
Krapfenbauer K, Engidawork E, Cairns N, Fountoulakis M, Lubec G (2003) Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res 967(1–2):152–160
Radak D, Resanovic I, Isenovic ER (2013) Link between oxidative stress and acute brain ischemia. Angiology. doi:10.1177/0003319713506516
Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA (2010) Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology 59(4–5):290–294. doi:10.1016/j.neuropharm.2010.04.005
Dumont M, Beal MF (2011) Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med 51(5):1014–1026. doi:10.1016/j.freeradbiomed.2010.11.026
Zhang G, Ghosh S (2000) Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J Endotoxin Res 6(6):453–457
Kosmidou I, Vassilakopoulos T, Xagorari A, Zakynthinos S, Papapetropoulos A, Roussos C (2002) Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. Am J Respir Cell Mol Biol 26(5):587–593. doi:10.1165/ajrcmb.26.5.4598
Camandola S, Mattson MP (2007) NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets 11(2):123–132. doi:10.1517/14728222.11.2.123
Qin ZH, Tao LY, Chen X (2007) Dual roles of NF-kappaB in cell survival and implications of NF-kappaB inhibitors in neuroprotective therapy. Acta Pharmacol Sin 28(12):1859–1872. doi:10.1111/j.1745-7254.2007.00741.x
Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M (1999) NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 5(5):554–559. doi:10.1038/8432
Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J (2004) Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke 35(4):987–991. doi:10.1161/01.STR.0000120732.45951.26
Cui L, Zhang X, Yang R, Liu L, Wang L, Li M, Du W (2010) Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacol Biochem Behav 96(4):469–475. doi:10.1016/j.pbb.2010.07.007
Wang L, Zhang X, Liu L, Cui L, Yang R, Li M, Du W (2010) Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res 1321:143–151. doi:10.1016/j.brainres.2009.12.046
Liu Y, Zhang XJ, Yang CH, Fan HG (2009) Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-kappaB expression. Brain Res 1268:174–180. doi:10.1016/j.brainres.2009.02.069
Dziennis S, Jia T, Ronnekleiv OK, Hurn PD, Alkayed NJ (2007) Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci 27(27):7268–7274. doi:10.1523/JNEUROSCI.1558-07.2007
Dziennis S, Alkayed NJ (2008) Role of signal transducer and activator of transcription 3 in neuronal survival and regeneration. Rev Neurosci 19(4–5):341–361
Xuan YT, Guo Y, Han H, Zhu Y, Bolli R (2001) An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A 98(16):9050–9055. doi:10.1073/pnas.161283798
Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R (2005) Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation 112(13):1971–1978. doi:10.1161/CIRCULATIONAHA.105.561522
Planas AM, Gorina R, Chamorro A (2006) Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem Soc Trans 34(Pt 6):1267–1270. doi:10.1042/BST0341267
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91
Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C (2004) Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 35(4):992–997. doi:10.1161/01.STR.0000119754.85848.0D
Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD (1986) A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke 17(4):738–743
Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, Dirnagl U (1998) Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 18(2):196–205. doi:10.1097/00004647-199802000-00011
Zhou R, Yang Z, Tang X, Tan Y, Wu X, Liu F (2013) Propofol protects against focal cerebral ischemia via inhibition of microglia-mediated proinflammatory cytokines in a rat model of experimental stroke. PLoS One 8(12):e82729. doi:10.1371/journal.pone.0082729
Vaibhav K, Shrivastava P, Javed H, Khan A, Ahmed ME, Tabassum R, Khan MM, Khuwaja G, Islam F, Siddiqui MS, Safhi MM, Islam F (2012) Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-kappaB in middle cerebral artery occlusion rat model. Mol Cell Biochem 367(1–2):73–84. doi:10.1007/s11010-012-1321-z
Maddahi A, Edvinsson L (2010) Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation 7:14. doi:10.1186/1742-2094-7-14
Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL (2005) Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci 233(1–2):125–132. doi:10.1016/j.jns.2005.03.022
de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P, Langhans W, Giannakopoulos P (2009) In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J Neurochem 110(1):12–22. doi:10.1111/j.1471-4159.2009.06098.x
Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E (2000) Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci 12(7):2265–2272
Hall ED, Braughler JM (1989) Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med 6(3):303–313
Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K (2008) Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett 180(3):222–229. doi:10.1016/j.toxlet.2008.06.869
Nadeem A, Siddiqui N, Alharbi NO, Alharbi MM, Imam F, Sayed-Ahmed MM (2014) Glutathione modulation during sensitization as well as challenge phase regulates airway reactivity and inflammation in mouse model of allergic asthma. Biochimie. doi:10.1016/j.biochi.2014.04.001
Benzi G, Moretti A (1995) Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol Aging 16(4):661–674
Mattia CJ, Adams JD Jr, Bondy SC (1993) Free radical induction in the brain and liver by products of toluene catabolism. Biochem Pharmacol 46(1):103–110
Qiao H, Dong L, Zhang X, Zhu C, Zhang X, Wang L, Liu Z, Chen L, Xing Y, Wang C, Li Y (2012) Protective effect of luteolin in experimental ischemic stroke: upregulated SOD1, CAT, Bcl-2 and claudin-5, down-regulated MDA and Bax expression. Neurochem Res 37(9):2014–2024. doi:10.1007/s11064-012-0822-1
Qiao H, Zhang X, Zhu C, Dong L, Wang L, Zhang X, Xing Y, Wang C, Ji Y, Cao X (2012) Luteolin downregulates TLR4, TLR5, NF-kappaB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res 1448:71–81. doi:10.1016/j.brainres.2012.02.003
Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP (1998) Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 18(9):3251–3260
Sambamurti K, Granholm AC, Kindy MS, Bhat NR, Greig NH, Lahiri DK, Mintzer JE (2004) Cholesterol and Alzheimer’s disease: clinical and experimental models suggest interactions of different genetic, dietary and environmental risk factors. Curr Drug Targets 5(6):517–528
Paris D, Patel N, Quadros A, Linan M, Bakshi P, Ait-Ghezala G, Mullan M (2007) Inhibition of Abeta production by NF-kappaB inhibitors. Neurosci Lett 415(1):11–16. doi:10.1016/j.neulet.2006.12.029
Echeverria V, Burgess S, Gamble-George J, Zeitlin R, Lin X, Cao C, Arendash GW (2009) Sorafenib inhibits nuclear factor kappa B, decreases inducible nitric oxide synthase and cyclooxygenase-2 expression, and restores working memory in APPswe mice. Neuroscience 162(4):1220–1231. doi:10.1016/j.neuroscience.2009.05.019
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G (2012) Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-kappaB-mediated inflammatory responses. PLoS One 7(11):e49701. doi:10.1371/journal.pone.0049701
Zhu J, Jiang Y, Wu L, Lu T, Xu G, Liu X (2012) Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience 202:342–351. doi:10.1016/j.neuroscience.2011.11.070
Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M (1999) Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem 274(13):8531–8538
Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L (2013) Electroacupuncture ameliorates cognitive impairment through inhibition of NF-kappaB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep 7(5):1516–1522. doi:10.3892/mmr.2013.1392
Campbell SJ, Anthony DC, Oakley F, Carlsen H, Elsharkawy AM, Blomhoff R, Mann DA (2008) Hepatic nuclear factor kappa B regulates neutrophil recruitment to the injured brain. J Neuropathol Exp Neurol 67(3):223–230. doi:10.1097/NEN.0b013e3181654957
Frantz S, Tillmanns J, Kuhlencordt PJ, Schmidt I, Adamek A, Dienesch C, Thum T, Gerondakis S, Ertl G, Bauersachs J (2007) Tissue-specific effects of the nuclear factor kappaB subunit p50 on myocardial ischemia-reperfusion injury. Am J Pathol 171(2):507–512. doi:10.2353/ajpath.2007.061042
Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH (2003) Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 23(8):3394–3406
Barreto G, White RE, Ouyang Y, Xu L, Giffard RG (2011) Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem 11(2):164–173
Gibson RM, Rothwell NJ, Le Feuvre RA (2004) CNS injury: the role of the cytokine IL-1. Vet J 168(3):230–237. doi:10.1016/j.tvjl.2003.10.016
Veerhuis R (2011) Histological and direct evidence for the role of complement in the neuroinflammation of AD. Curr Alzheimer Res 8(1):34–58
Kaltschmidt B, Kaltschmidt C (2009) NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol 1(3):a001271. doi:10.1101/cshperspect.a001271
Kaltschmidt B, Widera D, Kaltschmidt C (2005) Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta 1745(3):287–299. doi:10.1016/j.bbamcr.2005.05.009
Massa PT, Aleyasin H, Park DS, Mao X, Barger SW (2006) NFkappaB in neurons? The uncertainty principle in neurobiology. J Neurochem 97(3):607–618. doi:10.1111/j.1471-4159.2006.03810.x
Justicia C, Gabriel C, Planas AM (2000) Activation of the JAK/STAT pathway following transient focal cerebral ischemia: signaling through Jak1 and Stat3 in astrocytes. Glia 30(3):253–270
Zhou H, Zhang Z, Wei H, Wang F, Guo F, Gao Z, Marsicano G, Wang Q, Xiong L (2013) Activation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in rats. Brain Res 1529:154–164. doi:10.1016/j.brainres.2013.07.006
Israels LG, Israels ED (1999) Apoptosis. Oncologist 4(4):332–339
Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE (1993) Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262(5137):1274–1277
Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, Rosen CL, Huber JD, O’Callaghan JP (2010) Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience 170(2):633–644. doi:10.1016/j.neuroscience.2010.07.011
Lei C, Deng J, Wang B, Cheng D, Yang Q, Dong H, Xiong L (2011) Reactive oxygen species scavenger inhibits STAT3 activation after transient focal cerebral ischemia-reperfusion injury in rats. Anesth Analg 113(1):153–159. doi:10.1213/ANE.0b013e31821a9fbe
Di Domenico F, Casalena G, Jia J, Sultana R, Barone E, Cai J, Pierce WM, Cini C, Mancuso C, Perluigi M, Davis CM, Alkayed NJ, Butterfield DA (2012) Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion. J Neurochem 121(4):680–692. doi:10.1111/j.1471-4159.2012.07721.x
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean Government (MSIP; MRC, 2008–0062275), by a grant (A101836) from the Korean Health Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea.
Conflict of Interests
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig 1
Possible pathway. IL-32α reduce neuronal cell death as well as brain infarction through down regulate activation of NF-κB but up regulate activation of STAT3. (GIF 24 kb)
Rights and permissions
About this article
Cite this article
Hwang, C.J., Yun, HM., Jung, Y.Y. et al. Reducing Effect of IL-32α in the Development of Stroke Through Blocking of NF-κB, but Enhancement of STAT3 Pathways. Mol Neurobiol 51, 648–660 (2015). https://doi.org/10.1007/s12035-014-8739-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8739-0