Abstract
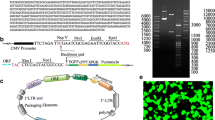
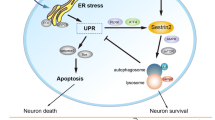
Following spinal cord injury (SCI), limit spontaneous functional recovery often emerged. However, the neuronal mechanisms associated with this phenomenon still remains obscure. By using proteomics analysis, endoplasmic reticulum protein 29 (ERp29) was discovered to increase in the motor cortexes of spinal cord transection (SCT) rats for 28 days post-operation (dpo) compared with in 14dpo. Then, the change in the expression of ERp29 was confirmed by using reverse transcription polymerase chain reaction (RT-PCR) and Western blot. To determine the role of ERp29 in the recovery of locomotor functions following SCT, lentiviral vectors were used to up- and downregulate the expression level of ERp29. Here, we found that cortical neurons in vitro with high level of ERp29 expression exhibited a significant proliferation, characterized by smaller size of soma and more extensive axon outgrowth, compared with neurons used as control, while ERp29 silence got the opposite results. In vivo, Lentivirus was inject into the cerebral cortex following SCT at thoracic level 10, which resulted in an increase number of neuronal nuclei(NeuN)-positive cells and less apoptotic cells. Moreover, increased PKC-γ immunoreactivity density was also found in the spinal cord T9 level compared with control rats. This was associated with a great functional improvement, indicated by Basso, Beattie, Bresnahan (BBB) locomotor rating scale. Lastly, we verified that ERp29 acts as a regulator by regulating a group of genes related with cell survival and apoptosis, involving in caspase and Erk, but not PI3K. Our findings showed that ERp29 can improve locomotor function by promoting neuronal survival and axonal regeneration in SCT rats via caspase and Erk signal pathway.








Similar content being viewed by others
References
Geisler FH, Dorsey FC, Coleman WP (1991) Recovery of motor function after spinal-cord injury-a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med 324(26):1829–1838
Yang HJ, Yang HJ, Yang XY et al (2009) Role of Neurotrophin 3 in spinal neuroplasticity in rats subjected to cord transection. Growth Factors 27(4):237–246
Li XL, Zhang W, Zhou X et al (2007) Temporal changes in the expression of some neurotrophins in spinal cord transected adult rats. Neuropeptides 41(3):135–143
Walczak CP, Tsai B (2011) A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J Virol 85(5):2386–2396
Hubbard MJ, McHugh NJ (2000) Human ERp29: isolation, primary structural characterisation and two-dimensional gel mapping. Electrophoresis 21(17):3785–3796
Hubbard MJ, McHugh NJ, Carne DL (2000) Isolation of ERp29, a novel endoplasmic reticulum protein, from rat enamel cells. Eur J Biochem 267(7):1945–1957
MacLeod JC, Sayer RJ, Lucocq JM et al (2004) ERp29, a general endoplasmic reticulum marker, is highly expressed throughout the brain. J Comp Neurol 477(1):29–42
Shimizu S, Brown M, Sengupta R et al (2011) CXCR7 protein expression in human adult brain and differentiated neurons. PLoS ONE 6(5):e20680
Park S, Hwang HM, Lee YH et al (2003) Expression of endoplasmic reticulum chaperone ERP29 in the injured spinal cord. Korean J Biol Sci 7(3):265–269
Baryshev M, Sargsyan E, Mkrtchian S (2006) ERp29 is an essential endoplasmic reticulum factor regulating secretion of thyroglobulin. Biochem Biophys Res Commun 340(2):617–624
Sargsyan E, Baryshev M, Szekely L et al (2002) Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J Biol Chem 277(19):17009–17015
Shang F, Zhao W, Zhao Q, et al (2012) Upregulation of eIF-5A1 in the paralysed muscle after spinal cord transection associates with spontaneous hindlimb locomotor recovery in rats by upregulation of the ErbB, MAPK and neurotrophin signal pathways. J Proteomics 91:188–199
Rainey-Barger EK, Mkrtchian S, Tsai B (2009) The C-terminal domain of ERp29 mediates polyomavirus binding, unfolding, and infection. J Virol 83(3):1483–1491
Magnuson B, Rainey EK, Benjamin T et al (2005) ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol Cell 20(2):289–300
Hoehenwarter W, Tang Y, Ackermann R et al (2008) Identification of proteins that modify cataract of mouse eye lens. Proteomics 8(23–24):5011–5024
Tuo J, Bojanowski CM, Zhou M et al (2007) Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci 48(8):3827–3836
Chan CC, Ross RJ, Shen D et al (2008) Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic Res 40(3–4):124–128
Verma V, Sauer T, Chan CC et al (2008) Constancy of ERp29 expression in cultured retinal pigment epithelial cells in the Ccl2/Cx3cr1 deficient mouse model of age-related macular degeneration. Curr Eye Res 33(8):701–707
Baryshev M, Sargsyan E, Wallin G et al (2004) Unfolded protein response is involved in the pathology of human congenital hypothyroid goiter and rat non-goitrous congenital hypothyroidism. J Mol Endocrinol 32(3):903–920
Lu H, Yang Y, Allister EM et al (2008) The identification of potential factors associated with the development of type 2 diabetes: a quantitative proteomics approach. Mol Cell Proteomics 7(8):1434–1451
Ying X, Liu Y, Guo Q, et al (2010) Endoplasmic reticulum protein 29 (ERp29), a protein related to sperm maturation is involved in sperm-oocyte fusion in mouse. Reprod Biol Endocrinol 8(10)
Guo W, Qu F, Xia L et al (2007) Identification and characterization of ERp29 in rat spermatozoa during epididymal transit. Reproduction 133(3):575–584
Zhang D, Richardson DR (2011) Endoplasmic reticulum protein 29 (ERp29): an emerging role in cancer. Int J Biochem Cell Biol 43(1):33–36
Gao D, Bambang IF, Putti TC et al (2011) ERp29 induces breast cancer cell growth arrest and survival through modulation of activation of p38 and upregulation of ER stress protein p58IPK. Lab Investig 92(2):200–213
Zhang D, Putti TC (2010) Over-expression of ERp29 attenuates doxorubicin-induced cell apoptosis through up-regulation of Hsp27 in breast cancer cells. Exp Cell Res 316(20):3522–3531
Bambang IF, Lee YK, Richardson DR et al (2012) Endoplasmic reticulum protein 29 regulates epithelial cell integrity during the mesenchymal–epithelial transition in breast cancer cells. Oncogene 32(10):1240–1251
Xu SG, Yan PJ, Shao ZM (2010) Differential proteomic analysis of a highly metastatic variant of human breast cancer cells using two-dimensional differential gel electrophoresis. J Cancer Res Clin Oncol 136(10):1545–1556
Bambang IF, Xu S, Zhou J et al (2009) Overexpression of endoplasmic reticulum protein 29 regulates mesenchymal–epithelial transition and suppresses xenograft tumor growth of invasive breast cancer cells. Lab Investig 89(11):1229–1242
Li X, Miyajima M, Mineki R et al (2005) Analysis of cerebellum proteomics in the hydrocephalic H-Tx rat. Neuroreport 16(6):571–574
Brea D, Rodríguez-González R, Sobrino T et al (2011) Proteomic analysis shows differential protein expression in endothelial progenitor cells between healthy subjects and ischemic stroke patients. Neurol Res 33(10):1057–1063
Tang B, Seredenina T, Coppola G et al (2011) Gene expression profiling of R6/2 transgenic mice with different CAG repeat lengths reveals genes associated with disease onset and progression in Huntington’s disease. Neurobiol Dis 42(3):459–467
Dukes AA, Van Laar VS, Cascio M et al (2008) Changes in endoplasmic reticulum stress proteins and aldolase A in cells exposed to dopamine. J Neurochem 106(1):333–346
Yu Z, Xu H, Zhang Y et al (2007) Expression of ERp29 in model of Parkinson’s disease of PC12 cells induced by proteasome inhibitor. J Jilin Univ (Med Ed) 2:020
Cavazzana-Calvo M, Payen E, Negre O et al (2010) Transfusion independence and hmga2 activation after gene therapy of human [bgr]-thalassaemia. Nature 467(7313):318–322
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76(2):319–370
Rossignol S, Drew T, Brustein E et al (1999) Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res 123:349–365
Wernig A, Müller S (1992) Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Spinal Cord 30(4):229–238
Dietz V, Wirz M, Curt A et al (1998) Locomotor pattern in paraplegic patients: training effects and recovery of spinal cord function. Spinal Cord 36(6):380–390
Farmaki E, Mkrtchian S, Papazian I et al (2011) ERp29 regulates response to doxorubicin by a PERK-mediated mechanism. Biochim Biophys Acta (BBA) - Mol Cell Res 1813(6):1165–1171
Huang YH, Chang AYW, Huang CM, et al (2002) Proteomic analysis of lipopolysaccharide‐induced apoptosis in PC12 cells. Proteomics 2(9): 1220–1228. 98
Zhang B, Wang M, Yang Y et al (2008) ERp29 is a radiation-responsive gene in IEC-6 cell. J Radiat Res 49(6):587–596
Guo L (2012) Endoplasmic reticulum protein ERp29 and doxorubicininduced toxicity in H9c2 cardiomyocytes: a comparative proteomics analysis. Asian Biomed 6(3)
Willis D, Li KW, Zheng JQ et al (2005) Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci 25(4):778–791
Bambang I, Lu D, Li H et al (2009) Cytokeratin 19 regulates endoplasmic reticulum stress and inhibits ERp29 expression via p38 MAPK/XBP-1 signaling in breast cancer cells. Exp Cell Res 315(11):1964–1974
Park S, You KH, Shong M et al (2005) Overexpression of ERp29 in the thyrocytes of FRTL-5 cells. Mol Biol Rep 32(1):7–13
Pang W, Leng X, Lu H et al (2013) Depletion of intracellular zinc induces apoptosis of cultured hippocampal neurons through suppression of ERK signaling pathway and activation of caspase-3. Neurosci Lett 552:140–145
Pang W, Lu H, Hu YD et al (2012) Depletion of intracellular zinc induced apoptosis in cultured hippocampal neurons through Raf/MEK/ERK pathways. Nutr Neurosci 15(1):18–24
Ma Y, Liu W, Wang Y et al (2011) VEGF protects rat cortical neurons from mechanical trauma injury induced apoptosis via the MEK/ERK pathway. Brain Res Bull 86(5):441–446
Anderson CNG, Tolkovsky AM (1999) A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci 19(2):664–673
Noh MY, Kim YS, Lee KY, et al (2013) The early activation of PI3K strongly enhances the resistance of cortical neurons to hypoxic injury via the activation of downstream targets of the PI3K pathway and the normalization of the levels of PARP activity, ATP, and NAD+. Mol Neurobiol 1–13
Dai R, Xia Y, Mao L et al (2012) Involvement of PI3K/Akt pathway in the neuroprotective effect of sonic hedgehog on cortical neurons under oxidative stress. J Huazhong Univ Sci Technol [Med Sci] 32:856–860
Zeng KW, Wang XM, Ko H et al (2011) Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid β-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur J Pharmacol 672(1):45–55
Torre AV, Junyent F, Folch J et al (2011) Study of the pathways involved in apoptosis induced by PI3K inhibition in cerebellar granule neurons. Neurochem Int 59(2):159–167
Wang W, Wang F, Liu J et al (2014) Snap25 ameliorates sensory deficit in rats with spinal cord transection. Mol Neurobiol. doi:10.1007/s12035-014-8642-8
Liu F, Zou Y, Liu S et al (2013) Electro-acupuncture treatment improves neurological function associated with downregulation of POGF and inhibition of asctrogliosis in rats with spinal cord transection. J Mol Neurosci 51(2):629–635
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, R., Zhao, W., Zhao, Q. et al. Endoplasmic Reticulum Protein 29 Protects Cortical Neurons From Apoptosis and Promoting Corticospinal Tract Regeneration to Improve Neural Behavior via Caspase and Erk Signal in Rats with Spinal Cord Transection. Mol Neurobiol 50, 1035–1048 (2014). https://doi.org/10.1007/s12035-014-8681-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8681-1