Abstract
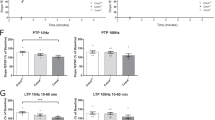
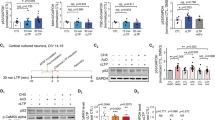
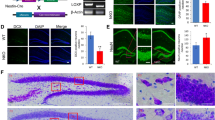
Maged1 is a member of the type II melanoma antigen (MAGE) family of proteins, which is highly conserved in the brain between mouse and human. Recently, Maged1 has been reported to be involved in depression and impaired sexual behavior. However, the role of Maged1 in learning and memory remains unknown. The aim of the present study was therefore to investigate whether Maged1 deficiency can impair learning and memory formation. By behavioral tests and electrophysiological recording, we observed that 5–6-month-old Maged1 knockout mice displayed the reduced basal synaptic transmission, pronounced hippocampal dysfunction, impaired spatial learning, and a deficit in long-term potentiation induction. Data from immunohistochemical and Western blot showed the reduced dendritic spine density and the number of synapses in the hippocampus of the Maged1 knockout mice, and Maged1 deficiency prevented the interaction of Maged1 with cAMP response element-binding protein (CREB). Furthermore, by chromatin immunoprecipitation and luciferase assay, we observed the downregulated activity of CREB and the suppressed CREB-dependent transcription after deficiency of Maged1, which lead to the decreased levels of brain-derived neurotrophic factor. Taken together, our results provide the evidence that Maged1 is involved in synaptic transmission and hippocampus-dependent learning and memory formation.






Similar content being viewed by others
References
Feng Y, Gao J, Yang M (2011) When MAGE meets RING: insights into biological functions of MAGE proteins. Protein Cell 2:7–12
Barker PA, Salehi A (2002) The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res 67:705–712
Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S (2001) An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res 61:5544–5551
Doyle JM, Gao J, Wang J, Yang M, Potts PR (2010) MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell 39:963–974
Goldman B, DeFrancesco L (2009) The cancer vaccine roller coaster. Nat Biotechnol 27:129–139
Ohman Forslund K, Nordqvist K (2001) The melanoma antigen genes—any clues to their functions in normal tissues? Exp Cell Res 265:185–194
Salehi AH, Roux PP, Kubu CJ, Zeindler C, Bhakar A, Tannis LL, Verdi JM, Barker PA (2000) NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor-dependent apoptosis. Neuron 27:279–288
Salehi AH, Xanthoudakis S, Barker PA (2002) NRAGE, a p75 neurotrophin receptor-interacting protein, induces caspase activation and cell death through a JNK-dependent mitochondrial pathway. J Biol Chem 277:48043–48050
Bragason BT, Palsdottir A (2005) Interaction of PrP with NRAGE, a protein involved in neuronal apoptosis. Mol Cell Neurosci 29:232–244
Di Certo MG, Corbi N, Bruno T, Iezzi S, De Nicola F, Desantis A, Ciotti MT, Mattei E, Floridi A, Fanciulli M, Passananti C (2007) NRAGE associates with the anti-apoptotic factor Che-1 and regulates its degradation to induce cell death. J Cell Sci 120:1852–1858
Kendall SE, Goldhawk DE, Kubu C, Barker PA, Verdi JM (2002) Expression analysis of a novel p75(NTR) signaling protein, which regulates cell cycle progression and apoptosis. Mech Dev 117:187–200
Nikopoulos GN, Martins JF, Adams TL, Karaczyn A, Adams D, Vary C, Oxburgh L, Verdi JM (2009) NRAGE: a potential rheostat during branching morphogenesis. Mech Dev 126:337–349
Calabrese G, Bennett BJ, Orozco L, Kang HM, Eskin E, Dombret C, De Backer O, Lusis AJ, Farber CR (2012) Systems genetic analysis of osteoblast-lineage cells. PLoS Genet 8:e1003150
Rochira JA, Matluk NN, Adams TL, Karaczyn AA, Oxburgh L, Hess ST, Verdi JM (2011) A small peptide modeled after the NRAGE repeat domain inhibits XIAP-TAB1-TAK1 signaling for NF-kappaB activation and apoptosis in P19 cells. PLoS One 6:e20659
Bertrand M, Huijbers I, Chomez P, De Backer O (2004) Comparative expression analysis of the MAGED genes during embryogenesis and brain development. Dev Dyn 230:325–334
Wang X, Tang J, Xing L, Shi G, Ruan H, Gu X, Liu Z, Wu X, Gao X, Xu Y (2010) Interaction of MAGED1 with nuclear receptors affects circadian clock function. EMBO J 29:1389–1400
Mouri A, Sasaki A, Watanabe K, Sogawa C, Kitayama S, Mamiya T, Miyamoto Y, Yamada K, Noda Y, Nabeshima T (2012) MAGE-D1 regulates expression of depression-like behavior through serotonin transporter ubiquitylation. J Neurosci 32:4562–4580
Dombret C, Nguyen T, Schakman O, Michaud JL, Hardin-Pouzet H, Bertrand MJ, De Backer O (2012) Loss of Maged1 results in obesity, deficits of social interactions, impaired sexual behavior and severe alteration of mature oxytocin production in the hypothalamus. Hum Mol Genet 21:4703–4717
Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109
Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL (2005) Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 48:635–646
Ji M, Dong L, Jia M, Liu W, Zhang M, Ju L, Yang J, Xie Z, Yang J (2014) Epigenetic enhancement of brain-derived neurotrophic factor signaling pathway improves cognitive impairments induced by isoflurane exposure in aged rats. Mol Neurobiol. doi:10.1007/s12035-014-8659-z
Rochira JA, Cowling RA, Himmelfarb JS, Adams TL, Verdi JM (2010) Mapping of NRAGE domains reveals clues to cell viability in BMP signaling. Apoptosis 15:63–70
Tian XX, Rai D, Li J, Zou C, Bai Y, Wazer D, Band V, Gao Q (2005) BRCA2 suppresses cell proliferation via stabilizing MAGE-D1. Cancer Res 65:4747–4753
Kendall SE, Battelli C, Irwin S, Mitchell JG, Glackin CA, Verdi JM (2005) NRAGE mediates p38 activation and neural progenitor apoptosis via the bone morphogenetic protein signaling cascade. Mol Cell Biol 25:7711–7724
Williams ME, Strickland P, Watanabe K, Hinck L (2003) UNC5H1 induces apoptosis via its juxtamembrane region through an interaction with NRAGE. J Biol Chem 278:17483–17490
Matsuda T, Suzuki H, Oishi I, Kani S, Kuroda Y, Komori T, Sasaki A, Watanabe K, Minami Y (2003) The receptor tyrosine kinase Ror2 associates with the melanoma-associated antigen (MAGE) family protein Dlxin-1 and regulates its intracellular distribution. J Biol Chem 278:29057–29064
Sasaki A, Masuda Y, Iwai K, Ikeda K, Watanabe K (2002) A RING finger protein Praja1 regulates Dlx5-dependent transcription through its ubiquitin ligase activity for the Dlx/Msx-interacting MAGE/Necdin family protein, Dlxin-1. J Biol Chem 277:22541–22546
Kuwajima T, Nishimura I, Yoshikawa K (2006) Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J Neurosci 26:5383–5392
Barrett GL, Greferath U, Barker PA, Trieu J, Bennie A (2005) Co-expression of the P75 neurotrophin receptor and neurotrophin receptor-interacting melanoma antigen homolog in the mature rat brain. Neuroscience 133:381–392
Mayr B, Montminy M (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2:599–609
Montminy M (1997) Transcriptional regulation by cyclic AMP. Annu Rev Biochem 66:807–822
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623
Montminy MR, Bilezikjian LM (1987) Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 328:175–178
Hoeffler JP, Meyer TE, Yun Y, Jameson JL, Habener JF (1988) Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science 242:1430–1433
Stevens CF (1994) CREB and memory consolidation. Neuron 13:769–770
Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu Rev Neurosci 21:127–148
Tully T, Bourtchouladze R, Scott R, Tallman J (2003) Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov 2:267–277
Frank DA, Greenberg ME (1994) CREB: a mediator of long-term memory from mollusks to mammals. Cell 79:5–8
Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T (1994) Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79:49–58
Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79:59–68
Lamprecht R, Hazvi S, Dudai Y (1997) cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci 17:8443–8450
Guzowski JF, McGaugh JL (1997) Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci U S A 94:2693–2698
Josselyn SA, Shi C, Carlezon WA Jr, Neve RL, Nestler EJ, Davis M (2001) Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci 21:2404–2412
Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL (2005) Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci 119:1125–1130
Perazzona B, Isabel G, Preat T, Davis RL (2004) The role of cAMP response element-binding protein in Drosophila long-term memory. J Neurosci 24:8823–8828
Yin JC, Del Vecchio M, Zhou H, Tully T (1995) CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81:107–115
Barco A, Marie H (2011) Genetic approaches to investigate the role of CREB in neuronal plasticity and memory. Mol Neurobiol 44:330–349
Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP (2003) Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci 23:6304–6314
Acknowledgments
We thank Dr. Ying Xu for kindly providing all kinds of plasmids of Maged1. This work is partially supported by the National Basic Research Program of China (2013CB835100, 2011CBA01104), National Natural Science Foundation of China (nos. 81222013 and 30871264), and Specialized Research Funds for the Doctoral Program of Higher Education (20070284042, 20110091120029) to J Gao. J-J Yang is supported by the National Natural Science Foundation of China (81271216) and A-L Xu by the National Natural Science Foundation of China (nos. 81200186 and 81171233) and Natural Science Foundation of Shandong (ZR2011BL026).
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Yang, B. Lai, and A. Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, J., Lai, B., Xu, A. et al. Maged1 Co-interacting with CREB Through a Hexapeptide Repeat Domain Regulates Learning and Memory in Mice. Mol Neurobiol 51, 8–18 (2015). https://doi.org/10.1007/s12035-014-8677-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8677-x