Abstract
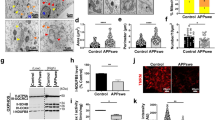
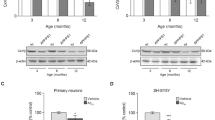
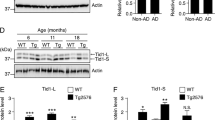
Mitochondrial dysfunction has been implicated in the pathogenesis of Alzheimer’s disease (AD). However, it is obscure how amyloid-beta (Aβ) can impair mitochondria in the early stage of AD pathology. Using PrP-hAPP/hPS1 double-transgenic AD mouse model, we find that abnormal mitochondrial morphology and damaged mitochondrial structure in hippocampal neurons appear in the early stage of AD-like disease development. We also find consistent mitochondrial abnormalities in the SH-SY5Y cells, which express amyloid precursor protein (APP) Swedish mutation (APPsw) and have been used as a cell model of the early-onset AD. Significant changes of mitofusin GTPases (Mfn1 and Mfn2) were detected both in the PrP-hAPP/hPS1 brains and SH-SY5Y cells. Moreover, our results show that Aβ accumulation in neurons of PrP-hAPP/hPS1 mice can affect the neurogenesis prior to plaque formation. These findings suggest that mitochondrial impairment is a very early event in AD pathogenesis and abnormal expression of Mfn1 and Mfn2 caused by excessive intracellular Aβ is the possible molecular mechanism. Interestingly, l-theanine has significant effects on regulating mitochondrial fusion proteins in SH-SY5Y (APPsw) cells. Overall, our results not only suggest a new early mechanism of AD pathogenesis but also propose a preventive candidate, l-theanine, for the treatment of AD.






Similar content being viewed by others
References
Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE (2009) Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 30(10):1574–1586. doi:10.1016/j.neurobiolaging.2007.12.005
Reddy PH (2009) Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp Neurol 218(2):286–292. doi:10.1016/j.expneurol.2009.03.042
Swerdlow RH, Khan SM (2009) The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol 218(2):308–315. doi:10.1016/j.expneurol.2009.01.011
Aliev G, Palacios HH, Walrafen B, Lipsitt AE, Obrenovich ME, Morales L (2009) Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int J Biochem Cell Biol 41(10):1989–2004. doi:10.1016/j.biocel.2009.03.015
Mancuso M, Coppede F, Murri L, Siciliano G (2007) Mitochondrial cascade hypothesis of Alzheimer’s disease: myth or reality? Antioxid Redox Signal 9(10):1631–1646. doi:10.1089/ars.2007.1761
Swerdlow RH, Khan SM (2004) A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses 63(1):8–20. doi:10.1016/j.mehy.2003.12.045
Scheffler K, Krohn M, Dunkelmann T, Stenzel J, Miroux B, Ibrahim S, von Bohlen Und Halbach O, Heinze HJ, Walker LC, Gsponer JA, Pahnke J (2012) Mitochondrial DNA polymorphisms specifically modify cerebral beta-amyloid proteostasis. Acta Neuropathol 124(2):199–208. doi:10.1007/s00401-012-0980-x
Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E (2010) Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol 119(5):523–541. doi:10.1007/s00401-010-0679-9
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8(7):499–509. doi:10.1038/nrn2168
Lin MT, Beal MF (2006) Alzheimer’s APP mangles mitochondria. Nat Med 12(11):1241–1243. doi:10.1038/nm1106-1241
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421, doi:S0896627303004343 [pii]
Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci 26(35):9057–9068. doi:10.1523/JNEUROSCI.1469-06.2006
Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH (2006) Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15(9):1437–1449. doi:10.1093/hmg/ddl066
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21(9):3017–3023, doi:21/9/3017 [pii]
Parker WD Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK (1994) Electron transport chain defects in Alzheimer’s disease brain. Neurology 44(6):1090–1096
Cardoso SM, Pereira CF, Moreira PI, Arduino DM, Esteves AR, Oliveira CR (2010) Mitochondrial control of autophagic lysosomal pathway in Alzheimer’s disease. Exp Neurol 223(2):294–298. doi:10.1016/j.expneurol.2009.06.008
Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA (2002) Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 80(1):91–100
Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA (2005) Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci 25(3):672–679. doi:10.1523/JNEUROSCI.4276-04.2005
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304(5669):448–452. doi:10.1126/science.1091230
Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M (2004) Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J Biol Chem 279(49):51654–51660. doi:10.1074/jbc.M404500200
Zhang J, Liu Q, Chen Q, Liu NQ, Li FL, Lu ZB, Qin C, Zhu H, Huang YY, He W, Zhao BL (2006) Nicotine attenuates beta-amyloid-induced neurotoxicity by regulating metal homeostasis. FASEB J Off Publ Fed Am Soc Exp Biol 20(8):1212–1214. doi:10.1096/fj.05-5214fje
Wan L, Nie G, Zhang J, Luo Y, Zhang P, Zhang Z, Zhao B (2011) beta-Amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic Biol Med 50(1):122–129. doi:10.1016/j.freeradbiomed.2010.10.707
Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4(1):97–100
Wang J, Tanila H, Puolivali J, Kadish I, van Groen T (2003) Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis 14(3):318–327
Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF (2005) Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med 202(9):1163–1169. doi:10.1084/jem.20051529
Wang H, He J, Zhang R, Zhu S, Wang J, Kong L, Tan Q, Li XM (2012) Sensorimotor gating and memory deficits in an APP/PS1 double transgenic mouse model of Alzheimer’s disease. Behav Brain Res 233(1):237–243. doi:10.1016/j.bbr.2012.05.007
Bao XQ, Li N, Wang T, Kong XC, Tai WJ, Sun H, Zhang D (2013) FLZ alleviates the memory deficits in transgenic mouse model of Alzheimer’s Disease via decreasing beta-amyloid production and tau hyperphosphorylation. PLoS One 8(11):e78033. doi:10.1371/journal.pone.0078033
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4(11):1313–1317. doi:10.1038/3305
Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Serrano J, Bentura ML, Martinez-Murillo R, Martinez A, Rodrigo J (2004) Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer’s disease. Histol Histopathol 19(3):823–844
Tong XK, Nicolakakis N, Fernandes P, Ongali B, Brouillette J, Quirion R, Hamel E (2009) Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiol Dis 35(3):406–414. doi:10.1016/j.nbd.2009.06.003
Wu Z, Zhang J, Zhao B (2009) Superoxide anion regulates the mitochondrial free Ca2+ through uncoupling proteins. Antioxid Redox Signal 11(8):1805–1818. doi:10.1089/ARS.2009.2427
Fink BD, Hong YS, Mathahs MM, Scholz TD, Dillon JS, Sivitz WI (2002) UCP2-dependent proton leak in isolated mammalian mitochondria. J Biol Chem 277(6):3918–3925. doi:10.1074/jbc.M107955200
Considine MJ, Goodman M, Echtay KS, Laloi M, Whelan J, Brand MD, Sweetlove LJ (2003) Superoxide stimulates a proton leak in potato mitochondria that is related to the activity of uncoupling protein. J Biol Chem 278(25):22298–22302. doi:10.1074/jbc.M301075200
Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB (2003) Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest 112(12):1831–1842. doi:10.1172/JCI19774
Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W (2006) Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A 103(49):18727–18732. doi:10.1073/pnas.0606298103
Di X, Yan J, Zhao Y, Zhang J, Shi Z, Chang Y, Zhao B (2010) L-theanine protects the APP (Swedish mutation) transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience 168(3):778–786. doi:10.1016/j.neuroscience.2010.04.019
Kokoszka JE, Coskun P, Esposito LA, Wallace DC (2001) Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A 98(5):2278–2283. doi:10.1073/pnas.051627098
Swerdlow RH (2007) Pathogenesis of Alzheimer’s disease. Clin Interv Aging 2(3):347–359
Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG (2003) Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161(1):41–54. doi:10.1083/jcb.200207030
Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM (2006) Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J Biol Chem 281(3):1599–1604. doi:10.1074/jbc.M507892200
Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK (2004) Oligomerization of Alzheimer’s beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci 24(14):3592–3599. doi:10.1523/JNEUROSCI.5167-03.2004
Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ (2000) The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry 39(35):10831–10839
Lazarov O, Marr RA (2010) Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol 223(2):267–281. doi:10.1016/j.expneurol.2009.08.009
Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, Trojanowski JQ, Blennow K (2010) Biological markers of amyloid beta-related mechanisms in Alzheimer’s disease. Exp Neurol 223(2):334–346. doi:10.1016/j.expneurol.2009.09.024
Breyhan H, Wirths O, Duan K, Marcello A, Rettig J, Bayer TA (2009) APP/PS1KI bigenic mice develop early synaptic deficits and hippocampus atrophy. Acta Neuropathol 117(6):677–685. doi:10.1007/s00401-009-0539-7
Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, Derks C, Dejaegere T, Pellegrini L, D'Hooge R, Scorrano L, De Strooper B (2006) Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126(1):163–175. doi:10.1016/j.cell.2006.06.021
Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79–99. doi:10.1146/annurev.cellbio.22.010305.104638
Shen T, Zheng M, Cao C, Chen C, Tang J, Zhang W, Cheng H, Chen KH, Xiao RP (2007) Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem 282(32):23354–23361. doi:10.1074/jbc.M702657200
Wappler EA, Institoris A, Dutta S, Katakam PV, Busija DW (2013) Mitochondrial dynamics associated with oxygen-glucose deprivation in rat primary neuronal cultures. PLoS One 8(5):e63206. doi:10.1371/journal.pone.0063206
Fu YJ, Xiong S, Lovell MA, Lynn BC (2009) Quantitative proteomic analysis of mitochondria in aging PS-1 transgenic mice. Cell Mol Neurobiol 29(5):649–664. doi:10.1007/s10571-009-9359-5
Aliev G, Seyidova D, Neal ML, Shi J, Lamb BT, Siedlak SL, Vinters HV, Head E, Perry G, Lamanna JC, Friedland RP, Cotman CW (2002) Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels as a central target for the development of human AD and AD-like pathology in aged transgenic mice. Ann N Y Acad Sci 977:45–64
Aliyev A, Chen SG, Seyidova D, Smith MA, Perry G, de la Torre J, Aliev G (2005) Mitochondria DNA deletions in atherosclerotic hypoperfused brain microvessels as a primary target for the development of Alzheimer’s disease. J Neurol Sci 229–230:285–292. doi:10.1016/j.jns.2004.11.040
Kim TI, Lee YK, Park SG, Choi IS, Ban JO, Park HK, Nam SY, Yun YW, Han SB, Oh KW, Hong JT (2009) l-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radic Biol Med 47(11):1601–1610. doi:10.1016/j.freeradbiomed.2009.09.008
Kakuda T (2011) Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction. Pharmacol Res 64(2):162–168. doi:10.1016/j.phrs.2011.03.010
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30930036, 30870587) and the Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences. We thank Lei Sun, Yan Teng (Institute of Biophysics, Chinese Academy of Sciences), and Cheng Yuan (Institute of Botany, Chinese Academy of Sciences) for their technical assistance. We thank Professor Lora Heisler (University of Aberdeen, UK) and Leandra R. Mangieri (University of Texas Health Science Center at Houston) for editing and proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Z., Zhu, Y., Cao, X. et al. Mitochondrial Toxic Effects of Aβ Through Mitofusins in the Early Pathogenesis of Alzheimer’s Disease. Mol Neurobiol 50, 986–996 (2014). https://doi.org/10.1007/s12035-014-8675-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8675-z