Abstract
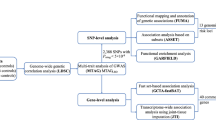
Amyotrophic lateral sclerosis (ALS) is the third most common neurodegenerative disease after Alzheimer’s disease (AD) and Parkinson’s disease (PD). In order to unravel more genetic etiology of ALS, genome-wide association studies (GWAS) have been conducted. However, the newly identified ALS susceptibility loci exert only very small risk effects and cannot fully explain the underlying ALS genetic risk. A large proportion of the heritability of ALS is still to be explained. Recently, pathway analysis of GWAS has been used to investigate the mechanisms of AD and PD. We think that AD or PD risk pathways may also be involved in ALS. In order to confirm this view, we conducted a pathway analysis of two independent ALS GWAS. We identified multiple classifications of the Kyoto Encyclopedia of Genes and Genomes pathways related to metabolism, immune system and diseases, environmental information processing, genetic information processing, cellular processes, and nervous system and neurodegenerative diseases to be the consistent signals in the two ALS GWAS. On the single pathway level, we identified 12 shared pathways. We compared the findings from ALS GWAS with those of previous pathway analyses of AD and PD GWAS. The results further supported the involvement of AD and PD risk pathways in ALS. We believe that our results may advance the understanding of ALS mechanisms and will be very useful for future genetic studies.
Similar content being viewed by others
References
Ahmeti KB, Ajroud-Driss S, Al-Chalabi A, Andersen PM, Armstrong J, Birve A, Blauw HM, Brown RH, Bruijn L, Chen W, Chio A, Comeau MC, Cronin S, Diekstra FP, Soraya Gkazi A, Glass JD, Grab JD, Groen EJ, Haines JL, Hardiman O, Heller S, Huang J, Hung WY, Jaworski JM, Jones A, Khan H, Landers JE, Langefeld CD, Leigh PN, Marion MC, McLaughlin RL, Meininger V, Melki J, Miller JW, Mora G, Pericak-Vance MA, Rampersaud E, Robberecht W, Russell LP, Salachas F, Saris CG, Shatunov A, Shaw CE, Siddique N, Siddique T, Smith BN, Sufit R, Topp S, Traynor BJ, Vance C, van Damme P, van den Berg LH, van Es MA, van Vught PW, Veldink JH, Yang Y, Zheng JG (2013) Age of onset of amyotrophic lateral sclerosis is modulated by a locus on 1p34.1. Neurobiol Aging 34(1):357 e357–319
Renton AE, Chio A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17(1):17–23
Schymick JC, Talbot K, Traynor BJ (2007) Genetics of sporadic amyotrophic lateral sclerosis. Hum Mol Genet 16 Spec No. 2:R233-242
Deng M, Wei L, Zuo X, Tian Y, Xie F, Hu P, Zhu C, Yu F, Meng Y, Wang H, Zhang F, Ma H, Ye R, Cheng H, Du J, Dong W, Zhou S, Wang C, Wang Y, Wang J, Chen X, Sun Z, Zhou N, Jiang Y, Liu X, Li X, Zhang N, Liu N, Guan Y, Han Y, Lv X, Fu Y, Yu H, Xi C, Xie D, Zhao Q, Xie P, Wang X, Zhang Z, Shen L, Cui Y, Yin X, Liang B, Zheng X, Lee TM, Chen G, Zhou F, Veldink JH, Robberecht W, Landers JE, Andersen PM, Al-Chalabi A, Shaw C, Liu C, Tang B, Xiao S, Robertson J, van den Berg LH, Sun L, Liu J, Yang S, Ju X, Wang K, Zhang X (2013) Genome-wide association analyses in Han Chinese identify two new susceptibility loci for amyotrophic lateral sclerosis. Nat Genet 45(6):697–700
Fogh I, Ratti A, Gellera C, Lin K, Tiloca C, Moskvina V, Corrado L, Soraru G, Cereda C, Corti S, Gentilini D, Calini D, Castellotti B, Mazzini L, Querin G, Gagliardi S, Del Bo R, Conforti FL, Siciliano G, Inghilleri M, Sacca F, Bongioanni P, Penco S, Corbo M, Sorbi S, Filosto M, Ferlini A, Di Blasio AM, Signorini S, Shatunov A, Jones A, Shaw PJ, Morrison KE, Farmer AE, Van Damme P, Robberecht W, Chio A, Traynor BJ, Sendtner M, Melki J, Meininger V, Hardiman O, Andersen PM, Leigh NP, Glass JD, Overste D, Diekstra FP, Veldink JH, van Es MA, Shaw CE, Weale ME, Lewis CM, Williams J, Brown RH, Landers JE, Ticozzi N, Ceroni M, Pegoraro E, Comi GP, D’Alfonso S, van den Berg LH, Taroni F, Al-Chalabi A, Powell J, Silani V (2014) A genome-wide association meta-analysis identifies a novel locus at 17q11.2 associated with sporadic amyotrophic lateral sclerosis. Hum Mol Genet. doi:10.1093/hmg/ddt587
Lambert JC, Grenier-Boley B, Chouraki V, Heath S, Zelenika D, Fievet N, Hannequin D, Pasquier F, Hanon O, Brice A, Epelbaum J, Berr C, Dartigues JF, Tzourio C, Campion D, Lathrop M, Amouyel P (2010) Implication of the immune system in Alzheimer’s disease: evidence from genome-wide pathway analysis. J Alzheimers Dis 20(4):1107–1118
Hong MG, Alexeyenko A, Lambert JC, Amouyel P, Prince JA (2010) Genome-wide pathway analysis implicates intracellular transmembrane protein transport in Alzheimer disease. J Hum Genet 55(10):707–709
Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, Pocklington A, Abraham R, Hollingworth P, Sims R, Gerrish A, Pahwa JS, Jones N, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, Heun R, Kolsch H, van den Bussche H, Heuser I, Peters O, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Ruther E, Carrasquillo MM, Pankratz VS, Younkin SG, Hardy J, O’Donovan MC, Owen MJ, Williams J (2010) Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One 5(11):e13950
Liu G, Jiang Y, Wang P, Feng R, Jiang N, Chen X, Song H, Chen Z (2012) Cell adhesion molecules contribute to Alzheimer’s disease: multiple pathway analyses of two genome-wide association studies. J Neurochem 120(1):190–198
Ramanan VK, Kim S, Holohan K, Shen L, Nho K, Risacher SL, Foroud TM, Mukherjee S, Crane PK, Aisen PS, Petersen RC, Weiner MW, Saykin AJ (2012) Genome-wide pathway analysis of memory impairment in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort implicates gene candidates, canonical pathways, and networks. Brain Imaging Behav 6(4):634–648
Liu G, Yao L, Liu J, Jiang Y, Ma G, Chen Z, Zhao B, Li K (2014) Cardiovascular disease contributes to Alzheimer’s disease: evidence from large-scale genome-wide association studies. Neurobiol Aging 35(4):786–792
Song GG, Lee YH (2013) Pathway analysis of genome-wide association studies for Parkinson’s disease. Mol Biol Rep 40(3):2599–2607
Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, Buchel F, Saad M, Bras JM, Bettella F, Nicolaou N, Simon-Sanchez J, Mittag F, Gibbs JR, Schulte C, Durr A, Guerreiro R, Hernandez D, Brice A, Stefansson H, Majamaa K, Gasser T, Heutink P, Wood NW, Martinez M, Singleton AB, Nalls MA, Hardy J, Morris HR, Williams NM (2014) A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum Mol Genet 23(2):562
Schymick JC, Scholz SW, Fung HC, Britton A, Arepalli S, Gibbs JR, Lombardo F, Matarin M, Kasperaviciute D, Hernandez DG, Crews C, Bruijn L, Rothstein J, Mora G, Restagno G, Chio A, Singleton A, Hardy J, Traynor BJ (2007) Genome-wide genotyping in amyotrophic lateral sclerosis and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol 6(4):322–328
Cronin S, Berger S, Ding J, Schymick JC, Washecka N, Hernandez DG, Greenway MJ, Bradley DG, Traynor BJ, Hardiman O (2008) A genome-wide association study of sporadic ALS in a homogenous Irish population. Hum Mol Genet 17(5):768–774
Hong MG, Pawitan Y, Magnusson PK, Prince JA (2009) Strategies and issues in the detection of pathway enrichment in genome-wide association studies. Hum Genet 126(2):289–301
Zhang B, Kirov S, Snoddy J (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33(Web Server issue):W741–748
Kim NC, Andrews PC, Asselbergs FW, Frost HR, Williams SM, Harris BT, Read C, Askland KD, Moore JH (2012) Gene ontology analysis of pairwise genetic associations in two genome-wide studies of sporadic ALS. BioData Min 5(1):9
Xie T, Deng L, Mei P, Zhou Y, Wang B, Zhang J, Lin J, Wei Y, Zhang X, Xu R (2014) A genome-wide association study combining pathway analysis for typical sporadic amyotrophic lateral sclerosis in Chinese Han populations. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2014.01.014
van Es MA, Veldink JH, Saris CG, Blauw HM, van Vught PW, Birve A, Lemmens R, Schelhaas HJ, Groen EJ, Huisman MH, van der Kooi AJ, de Visser M, Dahlberg C, Estrada K, Rivadeneira F, Hofman A, Zwarts MJ, van Doormaal PT, Rujescu D, Strengman E, Giegling I, Muglia P, Tomik B, Slowik A, Uitterlinden AG, Hendrich C, Waibel S, Meyer T, Ludolph AC, Glass JD, Purcell S, Cichon S, Nothen MM, Wichmann HE, Schreiber S, Vermeulen SH, Kiemeney LA, Wokke JH, Cronin S, McLaughlin RL, Hardiman O, Fumoto K, Pasterkamp RJ, Meininger V, Melki J, Leigh PN, Shaw CE, Landers JE, Al-Chalabi A, Brown RH Jr, Robberecht W, Andersen PM, Ophoff RA, van den Berg LH (2009) Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet 41(10):1083–1087
Shatunov A, Mok K, Newhouse S, Weale ME, Smith B, Vance C, Johnson L, Veldink JH, van Es MA, van den Berg LH, Robberecht W, Van Damme P, Hardiman O, Farmer AE, Lewis CM, Butler AW, Abel O, Andersen PM, Fogh I, Silani V, Chio A, Traynor BJ, Melki J, Meininger V, Landers JE, McGuffin P, Glass JD, Pall H, Leigh PN, Hardy J, Brown RH Jr, Powell JF, Orrell RW, Morrison KE, Shaw PJ, Shaw CE, Al-Chalabi A (2010) Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol 9(10):986–994
Acknowledgments
We thank Schymick and Cronin et al. for the ALS GWAS datasets. This work was supported by funding from the National Nature Science Foundation of China (grant numbers 81300945, 81171120, and 81100940).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hong Shang and Guiyou Liu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 62 kb)
Supplementary Table 2
(DOC 58 kb)
Rights and permissions
About this article
Cite this article
Shang, H., Liu, G., Jiang, Y. et al. Pathway Analysis of Two Amyotrophic Lateral Sclerosis GWAS Highlights Shared Genetic Signals with Alzheimer’s Disease and Parkinson’s Disease. Mol Neurobiol 51, 361–369 (2015). https://doi.org/10.1007/s12035-014-8673-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8673-1