Abstract
Oligomerisation of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes is required for synaptic vesicle fusion and neurotransmitter release. How these regulate the release of pain peptides elicited by different stimuli from sensory neurons has not been established. Herein, K+ depolarization was found to induce multiple sodium dodecyl sulfate (SDS)-resistant SNARE complexes in sensory neurons exposed to botulinum neurotoxins (BoNTs), with molecular weights ranging from 104–288 k (large) to 38–104 k (small). Isoform 1 of vesicle-associated membrane protein 1 (VAMP 1) assembled into stable complexes upon depolarisation and was required for the participation of intact synaptosome-associated protein of relative molecular mass 25 k (SNAP-25) or BoNT/A-truncated form (SNAP-25A) in the large functional and small inactive SDS-resistant SNARE complexes. Cleaving VAMP 1 decreased SNAP-25A in the functional complexes to a much greater extent than the remaining intact SNAP-25. Syntaxin 1 proved essential for the incorporation of intact and SNAP-25A into the large complexes. Truncation of syntaxin 1 by BoNT/C1 caused /A- and/or /C1-truncated SNAP-25 to appear in non-functional complexes and blocked the release of calcitonin gene-related peptide (CGRP) elicited by capsaicin, ionomycin, thapsigargin or K+ depolarization. Only the latter two were susceptible to /A. Inhibition of CGRP release by BoNT/A was reversed by capsaicin and/or ionomycin, an effect overcome by BoNT/C1. Unlike BoNT/B, BoNT/D cleaved VAMP 1 in addition to 2 and 3 in rat sensory neurons and blocked both CGRP and substance P release. Thus, unlike SNAP-25, syntaxin 1 and VAMP 1 are more suitable targets to abolish functional SNARE complexes and pain peptide release evoked by any stimuli.







Similar content being viewed by others
Abbreviations
- BoNT:
-
Botulinum neurotoxin
- CGNs:
-
Cerebellar granule neurons
- CGRP:
-
Calcitonin gene-related peptide
- DC:
-
Di-chain
- DMEM:
-
Dulbecco’s modified Eagle medium
- DRGs:
-
Dorsal root ganglionic neurons
- EIA:
-
Enzyme immunoassay
- HC:
-
Heavy chain
- LC:
-
Light chain
- LDCVs:
-
Large dense-core vesicles
- mAb:
-
Monoclonal antibody
- SC:
-
Single chain
- SNAP-25:
-
Synaptosome-associated protein of relative molecular mass 25 k
- SNARE complexes:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor complexes
- SP:
-
Substance P
- TGNs:
-
Trigeminal ganglionic neurons
- TRPV1:
-
Transient receptor potential vanilloid type 1
- VAMP 1 and 2:
-
Vesicle-associated membrane protein isoforms 1 and 2
References
Sudhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323(5913):474–477
Rothman JE, Warren G (1994) Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol 4(3):220–233
Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395(6700):347–353
Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE (1997) Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90(3):523–535
Otto H, Hanson PI, Jahn R (1997) Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci U S A 94(12):6197–6201
An SJ, Almers W (2004) Tracking SNARE complex formation in live endocrine cells. Science 306(5698):1042–1046
Lang T, Margittai M, Holzler H, Jahn R (2002) SNAREs in native plasma membranes are active and readily form core complexes with endogenous and exogenous SNAREs. J Cell Biol 158(4):751–760
van den Bogaart G, Holt MG, Bunt G, Riedel D, Wouters FS, Jahn R (2010) One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol 17(3):358–364
Shi L, Shen QT, Kiel A, Wang J, Wang HW, Melia TJ, Rothman JE, Pincet F (2012) SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science 335(6074):1355–1359
Jahn R, Scheller RH (2006) SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7(9):631–643
Hua Y, Scheller RH (2001) Three SNARE complexes cooperate to mediate membrane fusion. Proc Natl Acad Sci U S A 98(14):8065–8070
Montecucco C, Schiavo G, Pantano S (2005) SNARE complexes and neuroexocytosis: how many, how close? Trends Biochem Sci 30(7):367–372
Dolly JO, Wang J, Zurawski TH, Meng J (2011) Novel therapeutics based on recombinant botulinum neurotoxins to normalize the release of transmitters and pain mediators. FEBS J 278(23):4454–4466
Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H (1994) Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem 269(3):1617–1620
Schiavo G, Shone CC, Bennett MK, Scheller RH, Montecucco C (1995) Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem 270(18):10566–10570
Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R (1993) Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J 12(12):4821–4828
Schiavo G, Malizio C, Trimble WS, Polverino de Laureto P, Milan G, Sugiyama H, Johnson EA, Montecucco C (1994) Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J Biol Chem 269(32):20213–20216
Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, Cornille F, Roques B, Fykse EM, Sudhof TC, Jahn R et al (1994) Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J Biol Chem 269(17):12764–12772
Yamasaki S, Binz T, Hayashi T, Szabo E, Yamasaki N, Eklund M, Jahn R, Niemann H (1994) Botulinum neurotoxin type G proteolyses the Ala81-Ala82 bond of rat synaptobrevin 2. Biochem Biophys Res Commun 200(2):829–835
Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Sudhof TC, Niemann H (1994) Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J 13(21):5051–5061
Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H (1995) Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J 14(10):2317–2325
Skehel JJ, Wiley DC (1998) Coiled coils in both intracellular vesicle and viral membrane fusion. Cell 95(7):871–874
Chen YA, Scheller RH (2001) SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2(2):98–106
Wu Y, Gu Y, Morphew MK, Yao J, Yeh FL, Dong M, Chapman ER (2012) All three components of the neuronal SNARE complex contribute to secretory vesicle docking. J Cell Biol 198(3):323–330
Kubista H, Edelbauer H, Boehm S (2004) Evidence for structural and functional diversity among SDS-resistant SNARE complexes in neuroendocrine cells. J Cell Sci 117(Pt 6):955–966
Meng J, Wang J, Lawrence G, Dolly JO (2007) Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci 120(Pt 16):2864–2874
Durham PL, Cady R (2011) Insights into the mechanism of onabotulinumtoxinA in chronic migraine. Headache 51(10):1573–1577
Doods H, Arndt K, Rudolf K, Just S (2007) CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci 28(11):580–587
Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, Lawrence GW, Dolly JO (2009) Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci 29(15):4981–4992
Wang J, Zurawski TH, Meng J, Lawrence G, Olango WM, Finn DP, Wheeler L, Dolly JO (2011) A dileucine in the protease of botulinum toxin A underlies its long-lived neuroparalysis: transfer of longevity to a novel potential therapeutic. J Biol Chem 286(8):6375–6385
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41(1):207–234
Wang J, Meng J, Lawrence GW, Zurawski TH, Sasse A, Bodeker MO, Gilmore MA, Fernandez-Salas E, Francis J, Steward LE, Aoki KR, Dolly JO (2008) Novel chimeras of botulinum neurotoxins A and E unveil contributions from the binding, translocation, and protease domains to their functional characteristics. J Biol Chem 283(25):16993–17002
Wang J, Zurawski TH, Bodeker MO, Meng J, Boddul S, Aoki KR, Dolly JO (2012) Longer-acting and highly potent chimaeric inhibitors of excessive exocytosis created with domains from botulinum neurotoxin A and B. Biochem J 444(1):59–67
Malin SA, Davis BM, Molliver DC (2007) Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc 2(1):152–160
Lawrence GW, Dolly JO (2002) Multiple forms of SNARE complexes in exocytosis from chromaffin cells: effects of Ca2+, MgATP and botulinum toxin type A. J Cell Sci 115(Pt 3):667–673
Skofitsch G, Jacobowitz DM (1985) Calcitonin gene-related peptide coexists with substance P in capsaicin sensitive neurons and sensory ganglia of the rat. Peptides 6(4):747–754
Meng J, Wang J, Lawrence GW, Dolly JO (2013) Molecular components required for resting and stimulated endocytosis of botulinum neurotoxins by glutamatergic and peptidergic neurons. FASEB J 27(8):3167–3180
Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER (2006) SV2 is the protein receptor for botulinum neurotoxin A. Science 312(5773):592–596
Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET (2001) SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 294(5544):1117–1122
Liu Y, Sugiura Y, Lin W (2011) The role of synaptobrevin1/VAMP1 in Ca2+-triggered neurotransmitter release at the mouse neuromuscular junction. J Physiol 589(Pt 7):1603–1618
Huang PP, Khan I, Suhail MS, Malkmus S, Yaksh TL (2011) Spinal botulinum neurotoxin B: effects on afferent transmitter release and nociceptive processing. PLoS One 6(4):e19126
Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A (2009) Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 64(4):498–509
Zhao B, Wang HB, Lu YJ, Hu JW, Bao L, Zhang X (2011) Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res 21(5):741–753
Schiavo G, Stenbeck G, Rothman JE, Sollner TH (1997) Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc Natl Acad Sci U S A 94(3):997–1001
Sakaba T, Stein A, Jahn R, Neher E (2005) Distinct kinetic changes in neurotransmitter release after SNARE protein cleavage. Science 309(5733):491–494
Popoff MR, Poulain B (2010) Bacterial toxins and the nervous system: neurotoxins and multipotential toxins interacting with neuronal cells. Toxins (Basel) 2(4):683–737
Eleopra R, Montecucco C, Devigili G, Lettieri C, Rinaldo S, Verriello L, Pirazzini M, Caccin P, Rossetto O (2013) Botulinum neurotoxin serotype D is poorly effective in humans: an in vivo electrophysiological study. Clin Neurophysiol 124(5):999–1004
Pecze L, Blum W, Schwaller B (2013) Mechanism of capsaicin receptor TRPV1-mediated toxicity in pain-sensing neurons focusing on the effects of Na+/Ca2+ fluxes and the Ca2+-binding protein calretinin. Biochim Biophys Acta 1833:1680–1691
Wisnoskey BJ, Sinkins WG, Schilling WP (2003) Activation of vanilloid receptor type I in the endoplasmic reticulum fails to activate store-operated Ca2+ entry. Biochem J 372(Pt 2):517–528
Keller JE, Neale EA (2001) The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. J Biol Chem 276(16):13476–13482
Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A (1993) Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 57(3):845–859
Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R (2011) Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca2+ channel complex. Nat Med 17(7):822–829
Rigaud M, Gemes G, Weyker PD, Cruikshank JM, Kawano T, Wu HE, Hogan QH (2009) Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology 111(2):381–392
Gemes G, Bangaru ML, Wu HE, Tang Q, Weihrauch D, Koopmeiners AS, Cruikshank JM, Kwok WM, Hogan QH (2011) Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J Neurosci 31(10):3536–3549
Acknowledgments
This research is funded by a Principle Investigator Award (to J.O.D.) from Science Foundation Ireland which supports Dr. Meng and, in part, by Allergan Inc.
Conflict of Interest
We declare that this research is funded in part by Allergan Incorporated, P.O. Box 19534, Irvine, CA 92623, USA. The industrial sponsor had no role in study design, data collection and analysis or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
BoNT/D but not /B inhibited K+-evoked CGRP release from rat cultured DRG neurons, whereas both blocked its release from mouse DRGs. The latter were dissected from postnatal day 5 rats (open bars) or mice (hatched bars), dissociated and cultured for 7 days before being exposed to 100 nM recombinant BoNT/D, /B or toxin-free medium for 24 h at 37 °C; K+-evoked Ca2+-dependent CGRP release was assayed using EIA, as before, and plotted as % of the value for the respective toxin-free control. Results are means ± SEM; n ≥ 3 (PDF 11 kb)
Supplemental Fig. S2
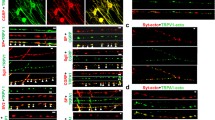
VAMP 1 is enriched in rat TGNs unlike central CGNs; VAMP 1 forms SNARE complexes with SNAP-25 and syntaxin 1 upon depolarisation. a Equal number of cells were lysed by SDS buffer for SDS-PAGE and Western blotting. Note that TGNs express more VAMP 1 than CGNs. b Upon stimulation of rat TGNs by HK, VAMP 1 was readily detectable in large (Mr of 104–288 k) SNARE complexes (PDF 60 kb)
Supplemental Fig. S3
The presence of VAMP 3 and a negligible amount of 2 in SDS resistant SNARE complexes. Rat TGNs were depolarized with high [K+] for 5 min at 37 °C before being lysed for 2-D electrophoresis. Antibodies against syntaxin 1, SNAP-25 and VAMP 2 were used for Western blotting. Note that VAMP 2 was hardly detected in SDS-resistant complex despite the presence of its free form (upper panel). Aliquots of the same samples were subjected to separate SDS-PAGE for probing VAMP 3 (lower panel). (PDF 91 kb)
Supplemental Fig. S4
In mouse TGNs, like rat, cleavage of syntaxin 1 leads SNAP-25C1 into small complexes. After cleaving syntaxin 1 and SNAP-25 with 100 nM/C1, cells were washed and stimulated with 60 mM K+ in the presence of 2.5 mM Ca2+ for 5 min, before lysis in SDS sample buffer for 2-D electrophoresis and immunoblot analysis. Note that the residual intact SNAP-25 resides predominantly in the large complexes whereas /C1-truncated SNAP-25 occurred in the small forms. Subsequent cleavage of VAMPs by /D reduced both complexes (PDF 178 kb)
Supplemental Fig. S5
Direct comparison of the levels of the two sizes of SNARE complexes in TGNs treated with BoNTs. After incubation with 100 nM toxins, the cells were depolarized with K+ for 5 min before being harvested in SDS buffer for 2-D electrophoresis. SNARE complexes of large (104–288 k) and small (38–104 k) sizes were analysed. a BoNT/D but not /B decreased the content of both sizes of SNARE complexes. b /C1 cleavage of syntaxin 1 resulted in the appearance of the majority of SNAP-25C1 in the small SNARE complexes; subsequent exposure to/D diminished the large and small SNARE complexes. c TGNs treated with BoNT/C1 followed by BoNT/A further reduced the intact SNAP-25 in the large complexes but did not preclude the presence of SNAP-25C1 in the smaller complexes; SNAP-25 cleaved by BoNT/A alone resided predominately in the large complexes. SNAP-25C1 occurs in the small rather than the large complexes after cleavage of syntaxin 1. d Treatment with /A followed by /D decreased both the large and small complexes (PDF 175 kb)
Rights and permissions
About this article
Cite this article
Meng, J., Dolly, J.O. & Wang, J. Selective Cleavage of SNAREs in Sensory Neurons Unveils Protein Complexes Mediating Peptide Exocytosis Triggered by Different Stimuli. Mol Neurobiol 50, 574–588 (2014). https://doi.org/10.1007/s12035-014-8665-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8665-1