Abstract
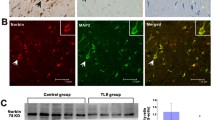
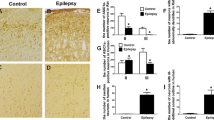
Efflux of monocaroxylates like lactate, pyruvate, and ketone bodies from astrocytes through monocarboxylate transporter 4 (MCT4) supplies the local neuron population with metabolic intermediates to meet energy requirements under conditions of increased demand. Disruption of this astroglial-neuron metabolic coupling pathway may contribute to epileptogenesis. We measured MCT4 expression in temporal lobe epileptic foci excised from patients with intractable epilepsy and in rats injected with pilocarpine, an animal model of temporal lobe epilepsy (TLE). Cortical MCT4 expression levels were significantly lower in TLE patients compared with controls, due at least partially to MCT4 promoter methylation. Expression of MCT4 also decreased progressively in pilocarpine-treated rats from 12 h to 14 days post-administration. Underexpression of MCT4 in cultured astrocytes induced by a short hairpin RNA promoted apoptosis. Knockdown of astrocyte MCT4 also suppressed excitatory amino acid transporter 1 (EAAT1) expression. Reduced MCT4 and EAAT1 expression by astrocytes may lead to neuronal hyperexcitability and epileptogenesis in the temporal lobe by reducing the supply of metabolic intermediates and by allowing accumulation of extracellular glutamate.








Similar content being viewed by others
References
Schurr A, Payne RS, Miller JJ, Rigor BM (1997) Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J Neurochem 69:423–426
Deitmer JW (2001) Strategies for metabolic exchange between glial cells and neurons. Respir Physiol 129:71–81
Cruz NF, Dienel GA (2002) High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab 22:1476–1489
Choi IY, Seaquist ER, Gruetter R (2003) Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res 72:25–32
Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR (2005) Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res 79:74–80
Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR (2005) Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res 81:644–652
Pellerin L, Magistretti PJ (2003) How to balance the brain energy budget while spending glucose differently. J Physiol 546:325
Pellerin L, Magistretti PJ (2004) Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 10:53–62
Dienel GA, Cruz NF (2004) Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int 45:321–351
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623:208–214
Schurr A, West CA, Rigor BM (1988) Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240:1326–1328
Izumi Y, Benz AM, Katsuki H, Zorumski CF (1997) Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J Neurosci 17:9448–9457
Juel C, Halestrap AP (1999) Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. J Physiol 517(Pt 3):633–642
Halestrap AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447:619–628
Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343(Pt 2):281–299
Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D (2011) Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci 31:538–548
Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ (2000) Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100:617–627
Pellerin L, Bergersen LH, Halestrap AP, Pierre K (2005) Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res 79:55–64
Cortes-Campos C, Elizondo R, Llanos P, Uranga RM, Nualart F, Garcia MA (2011) MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction. PLoS One 6:e16411
Gallagher SM, Castorino JJ, Wang D, Philp NJ (2007) Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res 67:4182–4189
Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, Eid T (2011) Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol Dis 41:577–584
Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, Wang Y, Bergersen LH, Eid T (2011) Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol Dis 45:165–176
Engel J Jr (1993) Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992). Neurology 43:1612–1617
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Smith E, De Young NJ, Tian ZQ, Caruso M, Ruszkiewicz AR, Liu JF, Jamieson GG, Drew PA (2008) Methylation of TIMP3 in esophageal squamous cell carcinoma. World J Gastroenterol: WJG 14:203–210
Liu B, Wang T, Wang L, Wang C, Zhang H, Gao GD (2011) Up-regulation of major vault protein in the frontal cortex of patients with intractable frontal lobe epilepsy. J Neurol Sci 308:88–93
Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G (2001) Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci 21:477–484
Turner DA, Adamson DC (2011) Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. J Neuropathol Exp Neurol 70:167–176
Levenson JM, Sweatt JD (2005) Epigenetic mechanisms in memory formation. Nat Rev Neurosci 6:108–118
Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD (2006) Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem 281:15763–15773
Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning and memory is associated with chromatin remodelling. Nature 447:178–182
Qureshi IA, Mehler MF (2010) Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis 39:53–60
Kobow K, Auvin S, Jensen F, Loscher W, Mody I, Potschka H, Prince D, Sierra A, Simonato M, Pitkanen A, Nehlig A, Rho JM (2012) Finding a better drug for epilepsy: antiepileptogenesis targets. Epilepsia 53:1868–1876
Kobow K, Blumcke I (2011) The methylation hypothesis: do epigenetic chromatin modifications play a role in epileptogenesis? Epilepsia 52(Suppl 4):15–19
Kobow K, Jeske I, Hildebrandt M, Hauke J, Hahnen E, Buslei R, Buchfelder M, Weigel D, Stefan H, Kasper B, Pauli E, Blumcke I (2009) Increased reelin promoter methylation is associated with granule cell dispersion in human temporal lobe epilepsy. J Neuropathol Exp Neurol 68:356–364
Choy MK, Movassagh M, Goh HG, Bennett MR, Down TA, Foo RS (2010) Genome-wide conserved consensus transcription factor binding motifs are hyper-methylated. BMC Genomics 11:519
Price NT, Jackson VN, Halestrap AP (1998) Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J 329(Pt 2):321–328
Meredith D, Christian HC (2008) The SLC16 monocaboxylate transporter family. Xenobiotica 38:1072–1106
Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, Wilson MC, Halestrap AP, Ottersen OP (2001) A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res Exp Hirnforsch Exp Cereb 136:523–534
Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L (2003) Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 122:677–688
Bergersen L, Rafiki A, Ottersen OP (2002) Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res 27:89–96
Shimada A, Nakagawa Y, Morishige H, Yamamoto A, Fujita T (2006) Functional characteristics of H+-dependent nicotinate transport in primary cultures of astrocytes from rat cerebral cortex. Neurosci Lett 392:207–212
Kobayashi M, Otsuka Y, Itagaki S, Hirano T, Iseki K (2006) Inhibitory effects of statins on human monocarboxylate transporter 4. Int J Pharm 317:19–25
Kobayashi M, Kagawa T, Narumi K, Itagaki S, Hirano T, Iseki K (2008) Bicarbonate supplementation as a preventive way in statins-induced muscle damage. J Pharm Pharm Sci 11:1–8
Lucas CA, Gillies RJ, Olson JE, Giuliano KA, Martinez R, Sneider JM (1988) Intracellular acidification inhibits the proliferative response in BALB/c-3 T3 cells. J Cell Physiol 136:161–167
Harris AB (1975) Cortical neuroglia in experimental epilepsy. Exp Neurol 49:691–715
Pollen DA, Trachtenberg MC (1970) Neuroglia: gliosis and focal epilepsy. Science 167:1252–1253
de Lanerolle NC, Lee TS, Spencer DD (2010) Astrocytes and epilepsy. Neurotherapeutics: J Am Soc Exp NeuroTher 7:424–438
Yamanishi S, Katsumura K, Kobayashi T, Puro DG (2006) Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol 290:H925–H934
Adijanto J, Philp NJ (2012) The SLC16A family of monocarboxylate transporters (MCTs)—physiology and function in cellular metabolism, pH homeostasis, and fluid transport. Curr Top Membr 70:275–311
Manning Fox JE, Meredith D, Halestrap AP (2000) Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol 529(Pt 2):285–293
Kekuda R, Manoharan P, Baseler W, Sundaram U (2013) Monocarboxylate 4 mediated butyrate transport in a rat intestinal epithelial cell line. Dig Dis Sci 58(3):660–667
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte–neuron lactate transport is required for long-term memory formation. Cell 144:810–823
Ivanov A, Zilberter Y (2011) Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenerg 3:9
Holmgren CD, Mukhtarov M, Malkov AE, Popova IY, Bregestovski P, Zilberter Y (2010) Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex in vitro. J Neurochem 112:900–912
Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y (2010) Metabolic control of vesicular glutamate transport and release. Neuron 68:99–112
Pan JW, Bebin EM, Chu WJ, Hetherington HP (1999) Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia 40:703–707
Bough K (2008) Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia 49(Suppl 8):91–93
Williamson A, Patrylo PR, Pan J, Spencer DD, Hetherington H (2005) Correlations between granule cell physiology and bioenergetics in human temporal lobe epilepsy. Brain 128:1199–1208
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Olney JW, Collins RC, Sloviter RS (1986) Excitotoxic mechanisms of epileptic brain damage. Adv Neurol 44:857–877
Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM (2005) Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol 57:226–235
Ahlemeyer B, Kehr K, Richter E, Hirz M, Baumgart-Vogt E, Herden C (2013) Phenotype, differentiation, and function differ in rat and mouse neocortical astrocytes cultured under the same conditions. J Neurosci Methods 212:156–164
Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702
Acknowledgments
The authors sincerely thank the patients and their families for their participation in this study. This work was supported by grants from the National Natural Science Foundation of China (no. 81271433). Funding organizations had no influence on the contents or on any other aspect of the article.
Conflict of Interest
The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bei Liu, Le Niu, Ming-Zhi Shen, and Lei Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, B., Niu, L., Shen, MZ. et al. Decreased Astroglial Monocarboxylate Transporter 4 Expression in Temporal Lobe Epilepsy. Mol Neurobiol 50, 327–338 (2014). https://doi.org/10.1007/s12035-013-8619-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8619-z